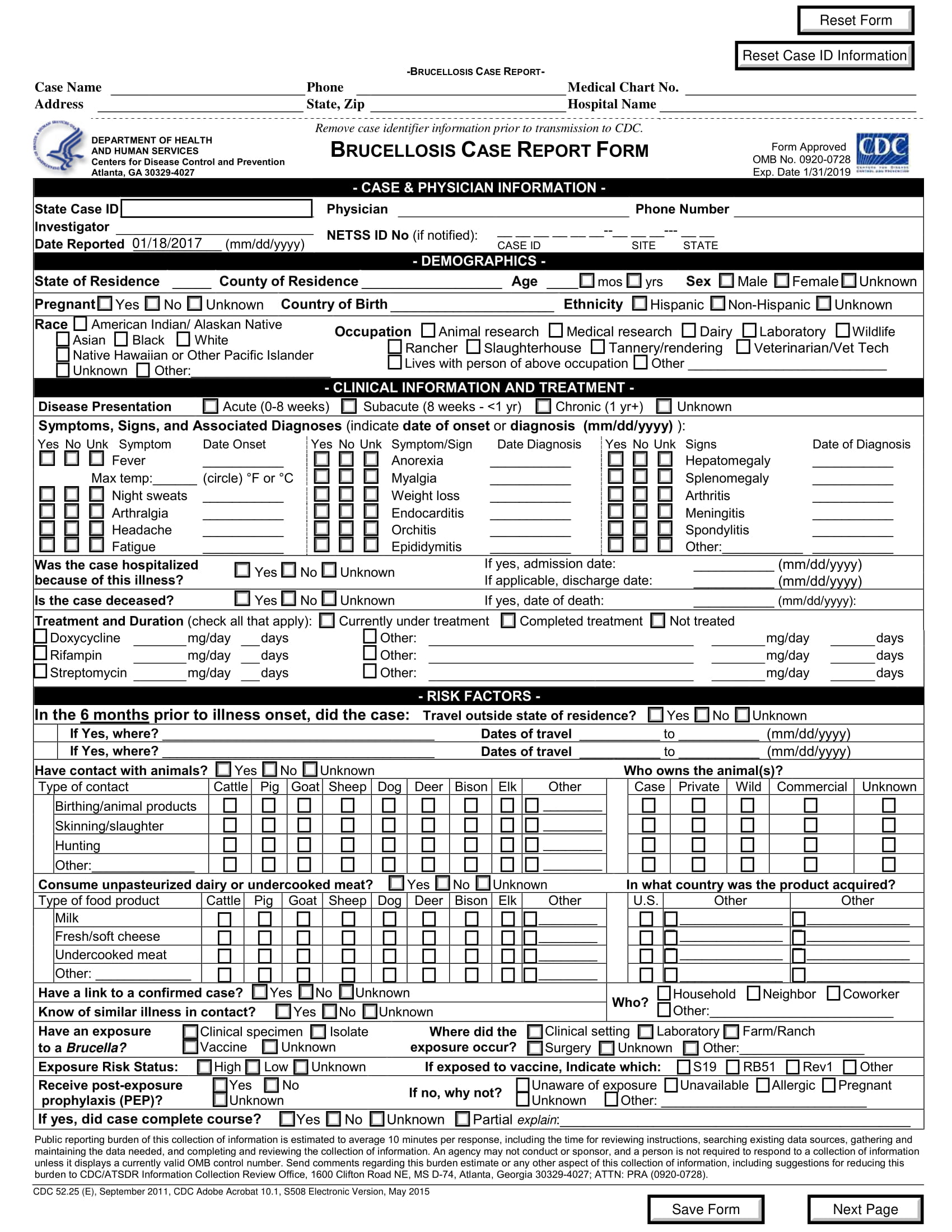
Irb Case Report Form . the various documents are grouped in three sections according to the stage of the trial during which they will normally be. 11/10/2023 this form is for use when a case report may directly or indirectly. case report form (crf) is a specialized document in clinical research. case report authorization form. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. It should be study protocol driven, robust in. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. The following protocol and consent templates are used by researchers in preparation for irb.
from www.sampleforms.com
the various documents are grouped in three sections according to the stage of the trial during which they will normally be. The following protocol and consent templates are used by researchers in preparation for irb. 11/10/2023 this form is for use when a case report may directly or indirectly. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. It should be study protocol driven, robust in. case report form (crf) is a specialized document in clinical research. case report authorization form. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is:
FREE 15+ Case Report Forms in PDF MS Word
Irb Case Report Form It should be study protocol driven, robust in. case report authorization form. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: 11/10/2023 this form is for use when a case report may directly or indirectly. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. It should be study protocol driven, robust in. case report form (crf) is a specialized document in clinical research. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. The following protocol and consent templates are used by researchers in preparation for irb.
From studylib.net
Clinical Trial Regulatory Maintenance CHAPTER10 10.1 Reporting to the IRB Irb Case Report Form a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: the various documents are grouped in three sections according to the stage of the trial during which they will normally be. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent.. Irb Case Report Form.
From www.signnow.com
Irb Proposal Template 20112024 Form Fill Out and Sign Printable PDF Irb Case Report Form the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. case report authorization form. for case reports or case series containing three or fewer patients, authors should. Irb Case Report Form.
From www.sampleforms.com
What Is a Case Report Form? [ Importance, Tips, Samples ] Irb Case Report Form a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: The following protocol and consent templates are used by researchers in preparation for irb. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. case report authorization form. the. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is:. Irb Case Report Form.
From www.pdffiller.com
IRB Applications and s Doc Template pdfFiller Irb Case Report Form the various documents are grouped in three sections according to the stage of the trial during which they will normally be. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. case report form (crf) is a specialized document in clinical research. for case reports or case series. Irb Case Report Form.
From studylib.net
IRB Annual / Continuing Review Form Irb Case Report Form the various documents are grouped in three sections according to the stage of the trial during which they will normally be. The following protocol and consent templates are used by researchers in preparation for irb. It should be study protocol driven, robust in. case report authorization form. 11/10/2023 this form is for use when a case report may. Irb Case Report Form.
From www.tpsearchtool.com
What Is A Case Report Form Importance Tips Samples Images Irb Case Report Form It should be study protocol driven, robust in. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. case report authorization form. 11/10/2023 this form is for use when a case report may directly or indirectly. the various documents are grouped in three sections according to the stage of. Irb Case Report Form.
From www.pdffiller.com
Case Study HIPAA Authorization (DOCX) Emory IRB Doc Template pdfFiller Irb Case Report Form for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. case report form (crf) is a specialized document in clinical research. case report authorization form. a case report. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form The following protocol and consent templates are used by researchers in preparation for irb. case report authorization form. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. 11/10/2023 this form is for use when a case report may directly or indirectly. a case report is a medical/educational. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form The following protocol and consent templates are used by researchers in preparation for irb. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. It should be study protocol driven, robust in. case report authorization form. for case reports or case series containing three or fewer. Irb Case Report Form.
From studylib.net
IRB Continuing Review/Final Report Application Irb Case Report Form a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: case report authorization form. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. 11/10/2023 this form is for use when a case report may directly or indirectly.. Irb Case Report Form.
From pray.gelorailmu.com
Basics Of Case Report Form Designing In Clinical Research with Case Irb Case Report Form case report authorization form. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. The following protocol and consent templates are used by researchers in preparation for irb. It should be study protocol driven, robust in. a case report is a medical/educational activity that does not. Irb Case Report Form.
From www.wordmstemplates.com
10+ Free Incident Report Templates Excel PDF Formats Irb Case Report Form It should be study protocol driven, robust in. 11/10/2023 this form is for use when a case report may directly or indirectly. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. the various documents are grouped in three sections according to the stage of the trial during which. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. 11/10/2023 this form is for use when a case report may directly or indirectly. The following protocol and consent. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. The following protocol and consent templates are used by researchers in preparation for irb. 11/10/2023 this form is. Irb Case Report Form.
From www.researchgate.net
A framework for collection of EMRbased RWD. CRF, case report form Irb Case Report Form case report authorization form. The following protocol and consent templates are used by researchers in preparation for irb. 11/10/2023 this form is for use when a case report may directly or indirectly. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. a case report is a medical/educational. Irb Case Report Form.
From www.researchgate.net
Clinical Trials Support Unit task delegation log. AE, adverse event Irb Case Report Form case report authorization form. The following protocol and consent templates are used by researchers in preparation for irb. case report form (crf) is a specialized document in clinical research. 11/10/2023 this form is for use when a case report may directly or indirectly. the various documents are grouped in three sections according to the stage of the. Irb Case Report Form.
From docest.com
Emory IRB Retrospective Chart Review Protocol Outline Docest Irb Case Report Form The following protocol and consent templates are used by researchers in preparation for irb. case report form (crf) is a specialized document in clinical research. 11/10/2023 this form is for use when a case report may directly or indirectly. the various documents are grouped in three sections according to the stage of the trial during which they will. Irb Case Report Form.
From studylib.net
IRB Reporting Form for Irb Case Report Form case report authorization form. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. It should be study protocol driven, robust in. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. 11/10/2023 this form is for use when a. Irb Case Report Form.
From www.yumpu.com
IRB Protocol Deviation Report Form This form is for IRB use only Irb Case Report Form 11/10/2023 this form is for use when a case report may directly or indirectly. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: The following protocol and consent templates are used by researchers in preparation for irb. It should be study protocol driven, robust in. case report form (crf). Irb Case Report Form.
From www.researchgate.net
Sample IRB information report for an individual clinical center. 15 Irb Case Report Form case report form (crf) is a specialized document in clinical research. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. 11/10/2023 this form is for use when a case. Irb Case Report Form.
From www.formsbank.com
Irb Consent Form Template printable pdf download Irb Case Report Form the various documents are grouped in three sections according to the stage of the trial during which they will normally be. case report authorization form. The following protocol and consent templates are used by researchers in preparation for irb. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is:. Irb Case Report Form.
From www.pdffiller.com
Merced College IRB, Adverse Incident Report Doc Template pdfFiller Irb Case Report Form 11/10/2023 this form is for use when a case report may directly or indirectly. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. It should be study protocol driven, robust in. The following protocol and consent templates are used by researchers in preparation for irb. the. Irb Case Report Form.
From studylib.net
Einstein IRB Reportable Event FollowUp Report Irb Case Report Form case report authorization form. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. It should be study protocol driven, robust in. case report form (crf) is a specialized. Irb Case Report Form.
From www.toptemplate.my.id
Case Report Form Template Clinical Trials Toptemplate.my.id Irb Case Report Form a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: case report authorization form. 11/10/2023 this form is for use when a case report may directly or indirectly. It should be study protocol driven, robust in. the various documents are grouped in three sections according to the stage of. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. case report form (crf) is a specialized document in clinical research. case report authorization form. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: It should be study. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. The following protocol and consent templates are used by researchers in preparation for irb. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. It should be study protocol. Irb Case Report Form.
From www.researchgate.net
Clinical Trials Support Unit task delegation log. AE, adverse event Irb Case Report Form case report form (crf) is a specialized document in clinical research. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: It should be study protocol driven, robust in. The following. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. case. Irb Case Report Form.
From www.xfanzexpo.com
Irb Hsr Status Forms Templates In Dsmb Report Template Professional Irb Case Report Form 11/10/2023 this form is for use when a case report may directly or indirectly. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. The following protocol and consent templates are used by researchers in preparation for irb. for case reports or case series containing three or. Irb Case Report Form.
From www.signnow.com
Case Report Format Complete with ease airSlate SignNow Irb Case Report Form case report form (crf) is a specialized document in clinical research. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. The following protocol and consent templates are. Irb Case Report Form.
From www.sampleforms.com
FREE 15+ Case Report Forms in PDF MS Word Irb Case Report Form the various documents are grouped in three sections according to the stage of the trial during which they will normally be. 11/10/2023 this form is for use when a case report may directly or indirectly. It should be study protocol driven, robust in. a case report is a medical/educational activity that does not meet the dhhs definition of. Irb Case Report Form.
From mungfali.com
Sample Case Report Form Irb Case Report Form the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. the various documents are grouped in three sections according to the stage of the trial during which they will normally be. The following protocol and consent templates are used by researchers in preparation for irb. It should be study protocol. Irb Case Report Form.
From studylib.net
IRB Application Form Irb Case Report Form 11/10/2023 this form is for use when a case report may directly or indirectly. the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: for case reports or case series containing. Irb Case Report Form.
From www.scribd.com
Case Study Valuation Report On IRB Infrastructure Limited PDF Irb Case Report Form a case report is a medical/educational activity that does not meet the dhhs definition of “research”, which is: the irb recommends the use of the consent templates to help researchers meet the legal requirements for consent. for case reports or case series containing three or fewer patients, authors should prepare an authorization form using the. It should. Irb Case Report Form.