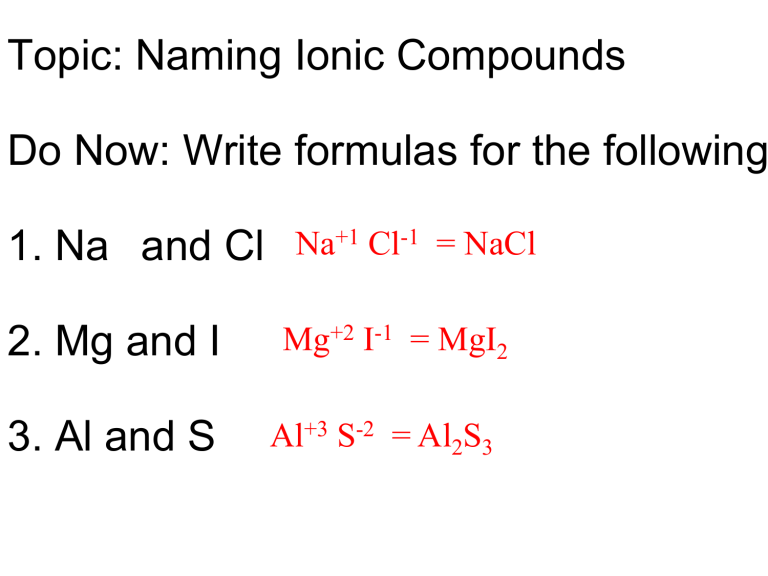
Aluminum Chlorine Ionic Compound Formula . between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. write the chemical formula for an ionic compound composed of each pair of ions. The magnesium ion and the. we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of.
from dxopqruud.blob.core.windows.net
we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. write the chemical formula for an ionic compound composed of each pair of ions. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. The magnesium ion and the. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence.
Aluminum Chloride Ionic Or Molecular at William Courtney blog
Aluminum Chlorine Ionic Compound Formula learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. write the chemical formula for an ionic compound composed of each pair of ions. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. The magnesium ion and the. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of.
From brunofuga.adv.br
Aluminium Chloride Formula Discounted Price brunofuga.adv.br Aluminum Chlorine Ionic Compound Formula learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. write the chemical formula for an ionic compound composed of each pair of ions. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive.. Aluminum Chlorine Ionic Compound Formula.
From www.youtube.com
How to Write the Formula for Aluminum chloride (AlCl3) YouTube Aluminum Chlorine Ionic Compound Formula learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary. Aluminum Chlorine Ionic Compound Formula.
From www.t3db.ca
T3DB Aluminium chloride Aluminum Chlorine Ionic Compound Formula write the chemical formula for an ionic compound composed of each pair of ions. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. the formula of. Aluminum Chlorine Ionic Compound Formula.
From slideplayer.com
Ionic formulas E Naming ions Forming compounds ppt download Aluminum Chlorine Ionic Compound Formula between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the. Aluminum Chlorine Ionic Compound Formula.
From www.adda247.com
Aluminium Chloride Formula AlCl3 Chemical Compound Name, Structure Aluminum Chlorine Ionic Compound Formula we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. learn how to write the chemical formula for a. Aluminum Chlorine Ionic Compound Formula.
From www.sliderbase.com
Naming Ionic Compounds Aluminum Chlorine Ionic Compound Formula learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the. Aluminum Chlorine Ionic Compound Formula.
From www.youtube.com
How to Balance Al + Cl2 = AlCl3 YouTube Aluminum Chlorine Ionic Compound Formula write the chemical formula for an ionic compound composed of each pair of ions. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. The magnesium ion and the. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence.. Aluminum Chlorine Ionic Compound Formula.
From www.youtube.com
How to Write the Formula for Aluminum chlorate YouTube Aluminum Chlorine Ionic Compound Formula between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. The magnesium ion and the. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. learn how to write the chemical formula for a simple ionic compound,. Aluminum Chlorine Ionic Compound Formula.
From www.youtube.com
How to Draw the Lewis Structure for AlCl3 Aluminum chloride YouTube Aluminum Chlorine Ionic Compound Formula learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. write the chemical formula for an ionic compound composed of each pair of ions. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive.. Aluminum Chlorine Ionic Compound Formula.
From www.numerade.com
SOLVEDWrite a formula for the ionic compound that forms between each pair of elements. a Aluminum Chlorine Ionic Compound Formula write the chemical formula for an ionic compound composed of each pair of ions. we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. the formula of an ionic compound represents the simplest ratio of the numbers of ions. Aluminum Chlorine Ionic Compound Formula.
From www.bharatagritech.com
What Is The Ionic Compound Formula Of Aluminum Chloride?, 47 OFF Aluminum Chlorine Ionic Compound Formula The magnesium ion and the. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. learn how to write the chemical formula for a simple ionic compound, such as. Aluminum Chlorine Ionic Compound Formula.
From www.slideserve.com
PPT Ch. 7 (Con’t.) Formula Writing & Naming of Compounds PowerPoint Presentation ID3835407 Aluminum Chlorine Ionic Compound Formula the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. The magnesium ion and the. learn why aluminum. Aluminum Chlorine Ionic Compound Formula.
From www.youtube.com
How to write Molecular formula of Aluminium chloride Chemical formula of Aluminium chloride Aluminum Chlorine Ionic Compound Formula we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. The magnesium ion and the. learn why aluminum. Aluminum Chlorine Ionic Compound Formula.
From www.slideserve.com
PPT Ionic Compounds PowerPoint Presentation, free download ID3730496 Aluminum Chlorine Ionic Compound Formula learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. The magnesium ion and the. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. the formula of an ionic compound represents the simplest ratio of the numbers. Aluminum Chlorine Ionic Compound Formula.
From www.numerade.com
SOLVEDWrite a formula for the ionic compound formed from each pair of elements. (a) Sodium and Aluminum Chlorine Ionic Compound Formula write the chemical formula for an ionic compound composed of each pair of ions. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. learn why aluminum. Aluminum Chlorine Ionic Compound Formula.
From brainly.in
Write down the formula of aluminium chloride by crisscross method, Brainly.in Aluminum Chlorine Ionic Compound Formula write the chemical formula for an ionic compound composed of each pair of ions. The magnesium ion and the. we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. between the aluminum and chlorine atoms there is a covalent. Aluminum Chlorine Ionic Compound Formula.
From dxopqruud.blob.core.windows.net
Aluminum Chloride Ionic Or Molecular at William Courtney blog Aluminum Chlorine Ionic Compound Formula between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. The magnesium ion and the. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. learn how to write the chemical formula for a simple ionic compound, such. Aluminum Chlorine Ionic Compound Formula.
From www.slideserve.com
PPT Ions, Ionic Bonds, and Metallic Bonds PowerPoint Presentation, free download ID2973921 Aluminum Chlorine Ionic Compound Formula learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. write the chemical formula for an ionic compound composed of each pair of ions. The magnesium ion and the. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer. Aluminum Chlorine Ionic Compound Formula.
From dxogyyswy.blob.core.windows.net
Aluminum Chloride Type Of Bond at Matthew Bean blog Aluminum Chlorine Ionic Compound Formula The magnesium ion and the. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. write the chemical formula for an ionic compound composed of each. Aluminum Chlorine Ionic Compound Formula.
From worksheetzonemasters.z19.web.core.windows.net
Formulas For Ionic Compounds Practice Aluminum Chlorine Ionic Compound Formula write the chemical formula for an ionic compound composed of each pair of ions. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. The magnesium ion and the. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the. Aluminum Chlorine Ionic Compound Formula.
From www.youtube.com
How to write chemical formula of Aluminium ChlorideMolecular formula YouTube Aluminum Chlorine Ionic Compound Formula learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. write the chemical formula for an ionic compound composed of each pair of ions. the. Aluminum Chlorine Ionic Compound Formula.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Aluminum Chlorine Ionic Compound Formula the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. The magnesium ion and the. learn why aluminum and chlorine form polar covalent bonds, not ionic. Aluminum Chlorine Ionic Compound Formula.
From www.slideserve.com
PPT Unit 6 Test Review PowerPoint Presentation, free download ID4134042 Aluminum Chlorine Ionic Compound Formula between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. write the chemical formula for an ionic compound composed of each pair of ions. the formula of. Aluminum Chlorine Ionic Compound Formula.
From www.slideserve.com
PPT Formulas for ionic compounds PowerPoint Presentation, free download ID5980526 Aluminum Chlorine Ionic Compound Formula write the chemical formula for an ionic compound composed of each pair of ions. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive.. Aluminum Chlorine Ionic Compound Formula.
From study.com
Aluminum Chloride AlCl3 Uses & Hazards Lesson Aluminum Chlorine Ionic Compound Formula between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. The magnesium ion and the. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and. Aluminum Chlorine Ionic Compound Formula.
From studylibraryopinion.z14.web.core.windows.net
How To Write Formula For Ionic Compounds Aluminum Chlorine Ionic Compound Formula learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. The magnesium ion and the. we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. write the chemical formula. Aluminum Chlorine Ionic Compound Formula.
From www.coursehero.com
[Solved] Predict the chemical formula for the ionic compound formed by the... Course Hero Aluminum Chlorine Ionic Compound Formula write the chemical formula for an ionic compound composed of each pair of ions. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. The magnesium ion and the. we could write the chemical formula for this ionic compound as mgclcl, but the convention is. Aluminum Chlorine Ionic Compound Formula.
From www.vrogue.co
Aluminium Chloride Formula Alcl3 Chemical Compound Na vrogue.co Aluminum Chlorine Ionic Compound Formula the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. between the aluminum and chlorine atoms there is a covalent bond with some degree. Aluminum Chlorine Ionic Compound Formula.
From www.adda247.com
Aluminium Chloride Formula AlCl3 Chemical Compound Name, Structure Aluminum Chlorine Ionic Compound Formula learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. write the chemical formula for an ionic compound composed of each pair of ions. . Aluminum Chlorine Ionic Compound Formula.
From www.slideserve.com
PPT IONIC COMPOUNDS Names and Formulas PowerPoint Presentation, free download ID2962564 Aluminum Chlorine Ionic Compound Formula we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. learn why aluminum and chlorine form polar covalent bonds, not ionic bonds, and how they form a dimer in the vapour phase. write the chemical formula for an ionic. Aluminum Chlorine Ionic Compound Formula.
From www.museoinclusivo.com
Is Aluminum Chloride Ionic or Covalent? An Analysis of Its Chemical Bonding Aluminum Profile Blog Aluminum Chlorine Ionic Compound Formula learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. The magnesium ion and the. learn how to write the chemical formula for a simple. Aluminum Chlorine Ionic Compound Formula.
From general.chemistrysteps.com
Naming Ionic Compounds Chemistry Steps Aluminum Chlorine Ionic Compound Formula between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. we could write the chemical formula for this ionic compound as mgclcl, but the convention is to use a numerical subscript when there is more than one ion. The magnesium ion and the. write the chemical formula for an. Aluminum Chlorine Ionic Compound Formula.
From schematicdiagramyakuza.z13.web.core.windows.net
Diagram Of Ionic Compound Aluminum Chlorine Ionic Compound Formula learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio of ions. write the chemical formula for an ionic compound composed of each pair of ions. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. the formula of an. Aluminum Chlorine Ionic Compound Formula.
From slideplayer.com
ppt download Aluminum Chlorine Ionic Compound Formula the formula of an ionic compound represents the simplest ratio of the numbers of ions necessary to give identical numbers of. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. write the chemical formula for an ionic compound composed of each pair of ions. The magnesium ion and. Aluminum Chlorine Ionic Compound Formula.
From ar.inspiredpencil.com
Aluminum Chloride Lewis Structure Aluminum Chlorine Ionic Compound Formula learn how to write the chemical formula for a simple ionic compound, such as sodium chloride (nacl), by balancing the positive. between the aluminum and chlorine atoms there is a covalent bond with some degree of charge separation (hence. learn how to write the chemical formula for a simple ionic compound, such as nacl, from the ratio. Aluminum Chlorine Ionic Compound Formula.