Endothermic Example Of Water . learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. Learn the difference between endothermic reactions and. When ammonium chloride (nh 4 cl) is dissolved in water, an. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water.
from www.slideserve.com
an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. When ammonium chloride (nh 4 cl) is dissolved in water, an. learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. Learn the difference between endothermic reactions and. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb.
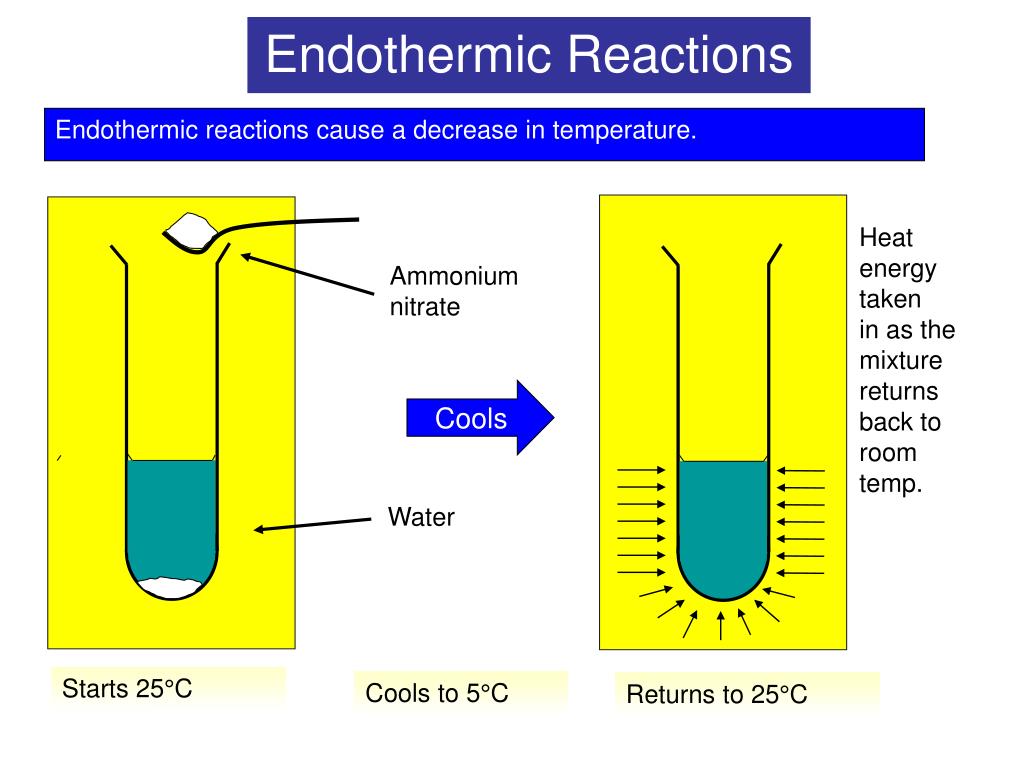
PPT Exothermic and endothermic reactions PowerPoint Presentation, free download ID5913704
Endothermic Example Of Water learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. When ammonium chloride (nh 4 cl) is dissolved in water, an. learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. Learn the difference between endothermic reactions and.
From stock.adobe.com
Activation energy in endothermic and exothermic reactions. Stock Illustration Adobe Stock Endothermic Example Of Water an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. When ammonium chloride (nh 4. Endothermic Example Of Water.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Example Of Water an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. When ammonium chloride (nh 4 cl) is dissolved in water, an. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. learn what an endothermic reaction is and how to. Endothermic Example Of Water.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. Learn the difference between endothermic reactions and. learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking. Endothermic Example Of Water.
From www.slideserve.com
PPT Exothermic and endothermic reactions PowerPoint Presentation, free download ID5913704 Endothermic Example Of Water learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. Learn the difference between endothermic reactions and. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. When ammonium chloride (nh 4 cl) is dissolved in water, an. . Endothermic Example Of Water.
From byjus.com
Difference Between Endothermic and Exothermic Reactions Chemistry Endothermic Example Of Water learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. Learn the difference between endothermic reactions and. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. an endothermic reaction is a chemical change in which the. Endothermic Example Of Water.
From mungfali.com
Endothermic Diagram With Labels Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. Learn the difference between endothermic reactions and. learn what. Endothermic Example Of Water.
From www.thoughtco.com
Endothermic Reaction Examples Endothermic Example Of Water an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. When ammonium. Endothermic Example Of Water.
From www.flexiprep.com
NCERT Class X Science Solutions Chapter 1 Chemical Reactions and Equations Part 2 FlexiPrep Endothermic Example Of Water learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. When ammonium chloride (nh 4 cl) is dissolved in water, an. Learn the difference between endothermic reactions and. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. an example of an. Endothermic Example Of Water.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Example Of Water an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. Learn the difference between endothermic reactions and. When ammonium chloride (nh 4 cl) is dissolved in water, an. . Endothermic Example Of Water.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Example Of Water an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. learn how to determine if a chemical process is exothermic or endothermic based on the. Endothermic Example Of Water.
From slideplayer.com
Exothermic and Endothermic Reactions ppt download Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. learn how to determine if a chemical process is exothermic or. Endothermic Example Of Water.
From slideplayer.com
Endothermic and Exothermic ppt download Endothermic Example Of Water an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. When ammonium chloride (nh 4 cl) is dissolved in water, an. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. an example of an easy endothermic reaction is dissolving potassium chloride. Endothermic Example Of Water.
From www.yaclass.in
Exothermic and Endothermic chemical changes — lesson. Science State Board, Class 7. Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. Learn the difference between endothermic reactions and. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. learn what an. Endothermic Example Of Water.
From www.numerade.com
SOLVED 'An example of an endothermic process is Subliming iodine crystals into gas b Sodium Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. Learn the difference between endothermic reactions. Endothermic Example Of Water.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Example Of Water learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. Learn the difference between endothermic reactions and. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. an endothermic reaction is a chemical reaction that absorbs. Endothermic Example Of Water.
From stock.adobe.com
Endothermic reactions list with external energy outline collection set. Educational labeled Endothermic Example Of Water learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings,. Endothermic Example Of Water.
From slideplayer.com
Notes on Four States of Matter ppt download Endothermic Example Of Water an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. When ammonium chloride (nh 4 cl) is dissolved in water, an. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. learn what an endothermic reaction is and how to. Endothermic Example Of Water.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Example Of Water learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. When ammonium chloride (nh 4 cl) is dissolved in water, an. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. learn what an endothermic reaction is and how to. Endothermic Example Of Water.
From worksheetcampusfires.z22.web.core.windows.net
Endothermic And Exothermic Activity Endothermic Example Of Water Learn the difference between endothermic reactions and. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. learn what an endothermic reaction is and see a list of examples of chemical and. Endothermic Example Of Water.
From www.bbc.co.uk
What are exothermic and endothermic reactions? BBC Bitesize Endothermic Example Of Water learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. Learn the difference between endothermic reactions and. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the. Endothermic Example Of Water.
From www.animalia-life.club
Endothermic And Exothermic Reaction Examples Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. an endothermic reaction is a chemical reaction that absorbs energy, mostly. Endothermic Example Of Water.
From byjus.com
State whether the following is exothermic or endothermic reaction and give reaso adding small Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. an endothermic reaction is a chemical change in which the system. Endothermic Example Of Water.
From www.tes.com
Endothermic and Exothermic Temperature Changes Edexcel 91 Teaching Resources Endothermic Example Of Water an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. When ammonium chloride (nh 4 cl) is dissolved in water, an. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. learn what an endothermic reaction is and how to perform a simple. Endothermic Example Of Water.
From www.pinterest.com
Endothermic and Exothermic Reactions infographic diagram showing relation between reactant Endothermic Example Of Water learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. Learn the difference between endothermic reactions and. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. learn what an endothermic reaction is and see a list of examples of. Endothermic Example Of Water.
From muadacsan3mien.com
What Are Exothermic And Endothermic Changes? Examples Unveiled! Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. an endothermic reaction is a chemical change in which the system. Endothermic Example Of Water.
From www.slideserve.com
PPT Endothermic Reaction Investigation Ammonium Chloride + Water PowerPoint Presentation Endothermic Example Of Water an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. . Endothermic Example Of Water.
From www.animalia-life.club
Endothermic Reaction Examples For Kids Endothermic Example Of Water an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. . Endothermic Example Of Water.
From classnotes123.com
What does one mean by exothermic and endothermic reactions? Give examples. CBSE Class Notes Endothermic Example Of Water an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. When ammonium chloride (nh 4 cl) is dissolved in water, an. learn how to determine if a. Endothermic Example Of Water.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Example Of Water learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. learn what an endothermic reaction is and see a list of examples of chemical and physical processes that absorb. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. Learn the. Endothermic Example Of Water.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Example Of Water When ammonium chloride (nh 4 cl) is dissolved in water, an. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. an endothermic reaction is a chemical reaction that absorbs. Endothermic Example Of Water.
From h-o-m-e.org
Endothermic Reactions The Science Behind Temperature Change Endothermic Example Of Water Learn the difference between endothermic reactions and. When ammonium chloride (nh 4 cl) is dissolved in water, an. an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its. Endothermic Example Of Water.
From slideplayer.com
Chapter Eighteen Energy and Reactions ppt download Endothermic Example Of Water an example of an easy endothermic reaction is dissolving potassium chloride (sold as a salt substitute) in your hand with water. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. When. Endothermic Example Of Water.
From manuallistbanderole.z13.web.core.windows.net
Energy Level Diagram For Endothermic Reaction Endothermic Example Of Water learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. Learn the difference between endothermic reactions. Endothermic Example Of Water.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Example Of Water Learn the difference between endothermic reactions and. an endothermic reaction is a chemical reaction that absorbs energy, mostly heat, from the surroundings. an endothermic reaction is a chemical change in which the system absorbs thermal energy from the surroundings, increasing its enthalpy. learn what an endothermic reaction is and see a list of examples of chemical and. Endothermic Example Of Water.
From ar.inspiredpencil.com
Endothermic And Exothermic Reaction Examples Endothermic Example Of Water Learn the difference between endothermic reactions and. learn what an endothermic reaction is and how to perform a simple and safe one with citric acid, baking soda, and. learn how to determine if a chemical process is exothermic or endothermic based on the enthalpy. learn what an endothermic reaction is and see a list of examples of. Endothermic Example Of Water.