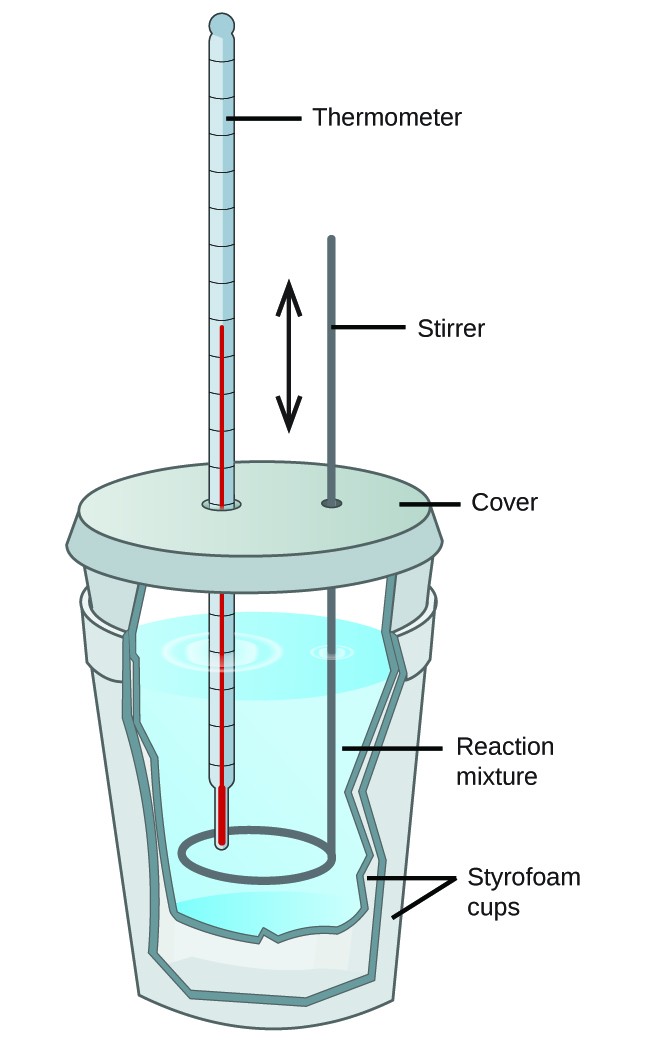
Calorimetry Description . what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic reaction.
from courses.lumenlearning.com
For example, when an exothermic reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process.
Calorimetry Chemistry Atoms First
Calorimetry Description calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to.
From brainly.in
Draw a neat labelled diagram for calorimeter Brainly.in Calorimetry Description what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the process of measuring the amount of. Calorimetry Description.
From www.studocu.com
Bomb Calorimetry lab description, this class has been in person if Calorimetry Description For example, when an exothermic reaction. what is calorimetry? calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is a branch of science concerned with measuring. Calorimetry Description.
From courses.lumenlearning.com
Calorimetry Chemistry Atoms First Calorimetry Description Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical. Calorimetry Description.
From physique.ensc-rennes.fr
TP calorimetry Calorimetry Description calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimetry Description.
From labsuppliesusa.com
Calorimeter Electric KLM Bio Scientific Calorimetry Description calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. For example, when. Calorimetry Description.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Calorimetry Description calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the process of measuring the amount of. Calorimetry Description.
From www.scribd.com
GC2 Thermodynamics and Calorimetry PDF Heat Calorimetry Calorimetry Description a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. For example, when an exothermic reaction. calorimetry is the process of measuring the amount of heat released or absorbed during. Calorimetry Description.
From www.studocu.com
Calorimetry Lab Calorimetry Lab Data and Results Table 1 Calorimetry Description what is calorimetry? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to.. Calorimetry Description.
From 2012books.lardbucket.org
Calorimetry Calorimetry Description calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic reaction. what is calorimetry? calorimetry is the measurement of the transfer. Calorimetry Description.
From www.scribd.com
lab 4 calorimetry lab Temperature Heat Capacity Calorimetry Description what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out. Calorimetry Description.
From www.medicalexpo.com
Isothermal titration calorimeter CCM Express® MGC Diagnostics Calorimetry Description calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a. Calorimetry Description.
From www.youtube.com
Bomb Calorimetry YouTube Calorimetry Description calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is the process of measuring the amount of heat released. Calorimetry Description.
From evulpo.com
evulpo Calorimetry Calorimetry Description what is calorimetry? For example, when an exothermic reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released. Calorimetry Description.
From www.dutcotennant.com
Differential Scanning Calorimeter (DSC) Dutco Tennant Calorimetry Description For example, when an exothermic reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. what is calorimetry? calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. a calorimeter is a device used to measure. Calorimetry Description.
From www.fishersci.com
Parr 6400 Automatic Isoperibol CalorimeterSpecialty Lab Equipment Calorimetry Description what is calorimetry? For example, when an exothermic reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimeter, device for measuring the heat developed during. Calorimetry Description.
From testbook.com
Principle of Calorimetry Definition, Formula, Uses, Examples Calorimetry Description calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. . Calorimetry Description.
From exoarnnpu.blob.core.windows.net
Calorimeter Description And Function at Kyla Ochs blog Calorimetry Description calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. what is calorimetry? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or. Calorimetry Description.
From www.labrotovap.com
Laboratory Digital Automatic Metal Bomb Calorimeter Lab Instrument Calorimetry Description Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic reaction. what is calorimetry? calorimetry is the measurement of the transfer of. Calorimetry Description.
From www.nanbiosis.com
U16. Surface Characterization and Calorimetry Unit Nanbiosis Calorimetry Description Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimetry Description.
From www.studocu.com
Calorimetry Lab Report 04/13/ CHEM 1300 Calorimetry PreLaboratory Calorimetry Description Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. For example, when an exothermic reaction. what is calorimetry? calorimetry is the measurement of the transfer of heat into or out. Calorimetry Description.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Description calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical. Calorimetry Description.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Calorimetry Description calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the.. Calorimetry Description.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Description what is calorimetry? a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. Calorimetry is a branch of science concerned with measuring a body’s state in. Calorimetry Description.
From www.youtube.com
Calorimetry / HEAT LECTURE 🌸 , NEXUS 2.0 Check Description for PDFs Calorimetry Description what is calorimetry? Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Calorimetry Description.
From users.highland.edu
Calorimetry Calorimetry Description calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic reaction. calorimeter, device for measuring the heat developed. Calorimetry Description.
From www.slideserve.com
PPT Bomb Calorimetry PowerPoint Presentation ID3206969 Calorimetry Description what is calorimetry? calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. Calorimetry Description.
From www.studocu.com
Calorimetry notes and descriptions Objects have the ability to absorb Calorimetry Description a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. what is calorimetry? calorimetry is the measurement of the transfer of heat into or out. Calorimetry Description.
From studylib.net
Calorimetry Worksheet Calorimetry Description what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. For example, when an exothermic reaction. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimeter, device for. Calorimetry Description.
From profilab24.com
IKA Calorimeter C 6000 isoperibol Package 2/12, without chiller Calorimetry Description For example, when an exothermic reaction. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the measurement of the transfer of heat into or. Calorimetry Description.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Description what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. For example, when an exothermic reaction. a calorimeter is a device used to. Calorimetry Description.
From www.youtube.com
Back to Basics Differential Scanning Calorimetry YouTube Calorimetry Description For example, when an exothermic reaction. what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is. Calorimetry Description.
From www.deltaed.co.nz
Calorimeter Joules w Electric Heating Delta Educational Calorimetry Description Calorimetry is a branch of science concerned with measuring a body’s state in terms of thermal features to. For example, when an exothermic reaction. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system. Calorimetry Description.
From slideplayer.com
Calorimetry. ppt download Calorimetry Description what is calorimetry? calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out. Calorimetry Description.
From www.intechopen.com
Applications of Calorimetry in a Wide Context Differential Scanning Calorimetry Description calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a. Calorimetry Description.
From www.youtube.com
MCAT Physics Temperature Vs Volume Graph Thermodynamics Calorimetry Description a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during. Calorimetry Description.