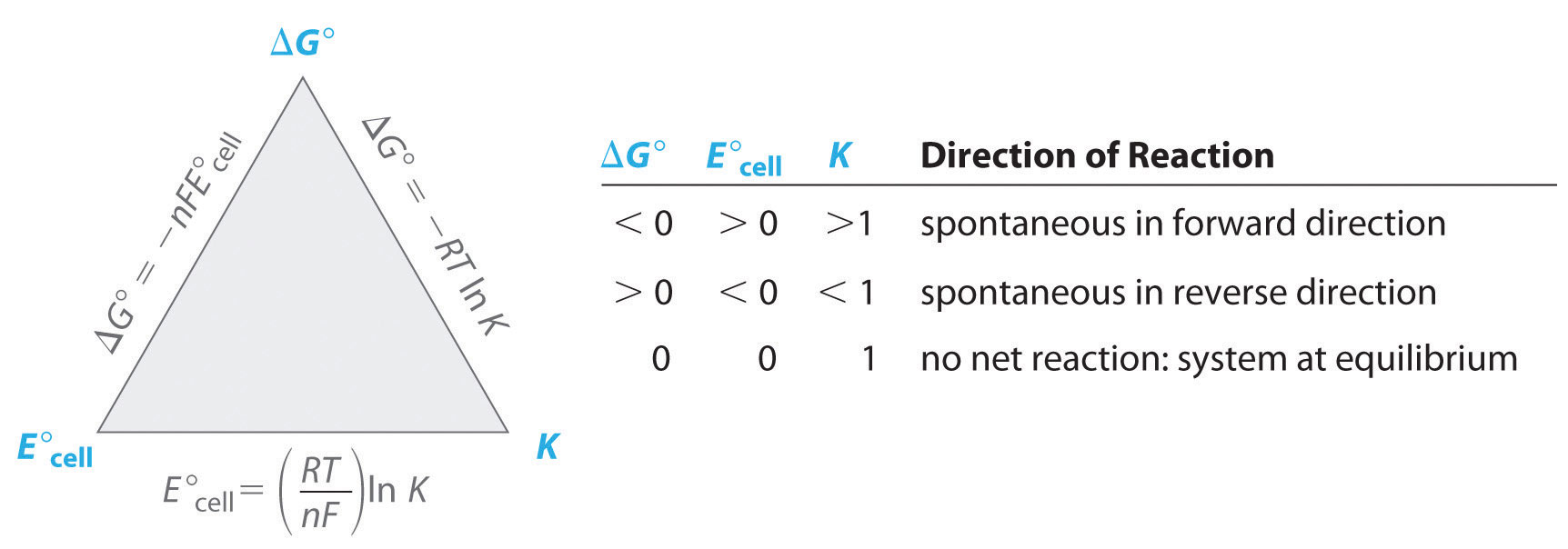
What Is K In Electrochemistry . Where n is the number of moles of. The nernst equation calculates electrochemical cell potential from. This movement of electrons is. electrochemistry is the study of chemical processes that cause electrons to move. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. Nernst equation to find equilibrium constant. The standard state potential for a cell. the actual relationship between the free energy change and the cell potential is given by the following formula: we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. to calculate the equilibrium constant for an electrochemical cell we need to know:
from saylordotorg.github.io
Nernst equation to find equilibrium constant. to calculate the equilibrium constant for an electrochemical cell we need to know: Where n is the number of moles of. electrochemistry is the study of chemical processes that cause electrons to move. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. This movement of electrons is. The nernst equation calculates electrochemical cell potential from. the actual relationship between the free energy change and the cell potential is given by the following formula: The standard state potential for a cell.
Electrochemistry
What Is K In Electrochemistry we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. electrochemistry is the study of chemical processes that cause electrons to move. The standard state potential for a cell. to calculate the equilibrium constant for an electrochemical cell we need to know: the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. the actual relationship between the free energy change and the cell potential is given by the following formula: This movement of electrons is. Nernst equation to find equilibrium constant. The nernst equation calculates electrochemical cell potential from. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. Where n is the number of moles of.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID2439047 What Is K In Electrochemistry Nernst equation to find equilibrium constant. The standard state potential for a cell. Where n is the number of moles of. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. The nernst equation calculates electrochemical cell potential from. electrochemistry is the study of chemical processes that cause. What Is K In Electrochemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is K In Electrochemistry This movement of electrons is. Where n is the number of moles of. to calculate the equilibrium constant for an electrochemical cell we need to know: Nernst equation to find equilibrium constant. The standard state potential for a cell. The nernst equation calculates electrochemical cell potential from. we can use the relationship between \delta {g^°} and the equilibrium. What Is K In Electrochemistry.
From www.youtube.com
Electrochemistry 06 Relating E, G, and K YouTube What Is K In Electrochemistry the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. The standard state potential for a cell. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. to calculate the equilibrium constant for an electrochemical cell we need to know:. What Is K In Electrochemistry.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field What Is K In Electrochemistry to calculate the equilibrium constant for an electrochemical cell we need to know: Where n is the number of moles of. The nernst equation calculates electrochemical cell potential from. Nernst equation to find equilibrium constant. electrochemistry is the study of chemical processes that cause electrons to move. we can use the relationship between \delta {g^°} and the. What Is K In Electrochemistry.
From schoolbag.info
Figure 12.3. The Cell Membrane as an Example of a Concentration Cell The electrochemical What Is K In Electrochemistry The standard state potential for a cell. to calculate the equilibrium constant for an electrochemical cell we need to know: Where n is the number of moles of. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. This movement of electrons is. Nernst equation to find equilibrium. What Is K In Electrochemistry.
From circuittawnilynne2461.z14.web.core.windows.net
Electrochemistry Cell Diagram What Is K In Electrochemistry The nernst equation calculates electrochemical cell potential from. Where n is the number of moles of. The standard state potential for a cell. Nernst equation to find equilibrium constant. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. to calculate the equilibrium constant for an electrochemical cell we need to. What Is K In Electrochemistry.
From www.youtube.com
What is Electrochemical Cell Notation Line notation Cell Diagram Notation Galvanic Cell What Is K In Electrochemistry the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. Nernst equation to find equilibrium constant. the actual relationship between the free energy change and the cell potential is given by the following formula: The nernst equation calculates electrochemical cell potential from. The standard state potential for a. What Is K In Electrochemistry.
From www.youtube.com
Electrochemistry, calculate K from E YouTube What Is K In Electrochemistry Nernst equation to find equilibrium constant. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. to calculate the equilibrium constant for an electrochemical cell we need to know: The nernst equation calculates electrochemical cell potential from. The standard state potential for a cell. the k value is very large,. What Is K In Electrochemistry.
From www.youtube.com
Electrochemistry 5 Nernst Equation Equilibrium Constant Gibb’s Free Energy Cell What Is K In Electrochemistry Where n is the number of moles of. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. to calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. electrochemistry is the study of chemical processes that cause. What Is K In Electrochemistry.
From www.youtube.com
What is Electrochemistry,definition(chemical part 1 for CBSE class 12 and JEE, IIT What Is K In Electrochemistry Nernst equation to find equilibrium constant. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. electrochemistry is the study of chemical processes that cause electrons to move. to calculate the equilibrium constant for an electrochemical cell we need to know: the k value is very large, indicating the. What Is K In Electrochemistry.
From exomnwzaf.blob.core.windows.net
What Is The Electrochemical Equivalent at Marcus Evenson blog What Is K In Electrochemistry the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. The nernst equation calculates electrochemical cell potential from. to calculate the equilibrium constant for an electrochemical cell we need to know: we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a. What Is K In Electrochemistry.
From www.vrogue.co
Electrochemistry Calculations Using The Nernst Equati vrogue.co What Is K In Electrochemistry to calculate the equilibrium constant for an electrochemical cell we need to know: we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. The nernst equation calculates electrochemical cell potential from. the actual relationship between the free energy change and the cell potential is given by the following formula: . What Is K In Electrochemistry.
From general.chemistrysteps.com
Cell Potential, Free Energy, and Equilibrium Constant Chemistry Steps What Is K In Electrochemistry we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. Nernst equation to find equilibrium constant. to calculate the equilibrium constant for an electrochemical cell we need to know: This movement of electrons is. the k value is very large, indicating the reaction proceeds to near completion to yield an. What Is K In Electrochemistry.
From www.slideshare.net
Chapter 6 electrochemistry What Is K In Electrochemistry Nernst equation to find equilibrium constant. The standard state potential for a cell. This movement of electrons is. to calculate the equilibrium constant for an electrochemical cell we need to know: Where n is the number of moles of. The nernst equation calculates electrochemical cell potential from. the actual relationship between the free energy change and the cell. What Is K In Electrochemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is K In Electrochemistry we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. The nernst equation calculates electrochemical cell potential from. Where n is the number of moles of. The standard state potential for a cell. the actual relationship between the free energy change and the cell potential is given by the following formula:. What Is K In Electrochemistry.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free download ID932862 What Is K In Electrochemistry The nernst equation calculates electrochemical cell potential from. Where n is the number of moles of. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. the actual relationship between. What Is K In Electrochemistry.
From 2012books.lardbucket.org
Electrochemistry What Is K In Electrochemistry The standard state potential for a cell. electrochemistry is the study of chemical processes that cause electrons to move. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. Nernst. What Is K In Electrochemistry.
From slideplayer.com
Electrochemistry. ppt download What Is K In Electrochemistry the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. Where n is the number of moles of. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. to calculate the equilibrium constant for an electrochemical cell we need to. What Is K In Electrochemistry.
From www.collegesearch.in
Electrochemical Cell Definitions, Examples, Electrochemistry, Classifications, Types, Salt What Is K In Electrochemistry Where n is the number of moles of. electrochemistry is the study of chemical processes that cause electrons to move. Nernst equation to find equilibrium constant. the actual relationship between the free energy change and the cell potential is given by the following formula: The nernst equation calculates electrochemical cell potential from. the k value is very. What Is K In Electrochemistry.
From dxozyitqu.blob.core.windows.net
What Is The Formula Of Electrochemistry at Alma Shufelt blog What Is K In Electrochemistry electrochemistry is the study of chemical processes that cause electrons to move. This movement of electrons is. Nernst equation to find equilibrium constant. the actual relationship between the free energy change and the cell potential is given by the following formula: The nernst equation calculates electrochemical cell potential from. The standard state potential for a cell. Where n. What Is K In Electrochemistry.
From saylordotorg.github.io
Electrochemistry What Is K In Electrochemistry the actual relationship between the free energy change and the cell potential is given by the following formula: Where n is the number of moles of. The standard state potential for a cell. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. we can use the. What Is K In Electrochemistry.
From www.slideserve.com
PPT Fundamentals of Electrochemistry PowerPoint Presentation, free download ID3358415 What Is K In Electrochemistry to calculate the equilibrium constant for an electrochemical cell we need to know: The nernst equation calculates electrochemical cell potential from. This movement of electrons is. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. electrochemistry is the study of chemical processes that cause electrons to move. The standard. What Is K In Electrochemistry.
From schoolbag.info
Electrochemical Cells Electrochemistry Training MCAT General Chemistry Review What Is K In Electrochemistry The nernst equation calculates electrochemical cell potential from. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. The standard state potential for a cell. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. Where n is the number of. What Is K In Electrochemistry.
From leverageedu.com
Electrochemical Series Notes Chemistry Class 11 & 12 Leverage Edu What Is K In Electrochemistry The nernst equation calculates electrochemical cell potential from. Where n is the number of moles of. to calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. This movement of electrons is.. What Is K In Electrochemistry.
From www.researchgate.net
a) Scheme of the electrochemical mechanism upon application of a... Download Scientific Diagram What Is K In Electrochemistry we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. The nernst equation calculates electrochemical cell potential from. Where n is the number of moles of. The standard state potential for. What Is K In Electrochemistry.
From scienceinfo.com
Electrochemistry Definition, Types, Components What Is K In Electrochemistry the actual relationship between the free energy change and the cell potential is given by the following formula: The nernst equation calculates electrochemical cell potential from. This movement of electrons is. Nernst equation to find equilibrium constant. electrochemistry is the study of chemical processes that cause electrons to move. the k value is very large, indicating the. What Is K In Electrochemistry.
From www.slideserve.com
PPT 21 Electrochemistry PowerPoint Presentation, free download ID826545 What Is K In Electrochemistry we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. Nernst equation to find equilibrium constant. the actual relationship between the free energy change and the cell potential is given by the following formula: the k value is very large, indicating the reaction proceeds to near completion to yield an. What Is K In Electrochemistry.
From www.youtube.com
AP Chemistry Electrochemistry Relating E, G, and K YouTube What Is K In Electrochemistry electrochemistry is the study of chemical processes that cause electrons to move. The standard state potential for a cell. The nernst equation calculates electrochemical cell potential from. Where n is the number of moles of. Nernst equation to find equilibrium constant. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium. What Is K In Electrochemistry.
From studylib.net
Introduction to Electrochemistry What Is K In Electrochemistry to calculate the equilibrium constant for an electrochemical cell we need to know: we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. Nernst equation to find equilibrium constant. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. . What Is K In Electrochemistry.
From slideplayer.com
Electrochemistry. ppt download What Is K In Electrochemistry The standard state potential for a cell. This movement of electrons is. electrochemistry is the study of chemical processes that cause electrons to move. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. to calculate the equilibrium constant for an electrochemical cell we need to know:. What Is K In Electrochemistry.
From chemistry.analia-sanchez.net
Electrochemistry Study Guide Chemistry Classes / Ronald Reagan S.H.S. What Is K In Electrochemistry the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. The standard state potential for a cell. This movement of electrons is. The nernst equation calculates electrochemical cell potential from. Where n is the number of moles of. Nernst equation to find equilibrium constant. we can use the. What Is K In Electrochemistry.
From www.youtube.com
Electrochemistry K. C. S. E Chemistry Paper 1 2013 2017 YouTube What Is K In Electrochemistry The nernst equation calculates electrochemical cell potential from. Nernst equation to find equilibrium constant. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. This movement of electrons is. The standard state potential for a cell. Where n is the number of moles of. to calculate the equilibrium constant for an. What Is K In Electrochemistry.
From 2012books.lardbucket.org
Electrochemistry What Is K In Electrochemistry the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly. The nernst equation calculates electrochemical cell potential from. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. Nernst equation to find equilibrium constant. The standard state potential for a cell.. What Is K In Electrochemistry.
From saylordotorg.github.io
Electrochemistry What Is K In Electrochemistry to calculate the equilibrium constant for an electrochemical cell we need to know: The standard state potential for a cell. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. the k value is very large, indicating the reaction proceeds to near completion to yield an equilibrium mixture containing mostly.. What Is K In Electrochemistry.
From www.slideserve.com
PPT electrochemistry PowerPoint Presentation, free download ID2683693 What Is K In Electrochemistry electrochemistry is the study of chemical processes that cause electrons to move. Where n is the number of moles of. Nernst equation to find equilibrium constant. we can use the relationship between \delta {g^°} and the equilibrium constant k, to obtain a relationship. the k value is very large, indicating the reaction proceeds to near completion to. What Is K In Electrochemistry.