Zinc Sulfate Soluble Or Insoluble . In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. If its solubility is greater than 0.1 mol/l, we call it soluble. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. Is zinc sulfate soluble in water? This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. Acidic salts, such as zinc sulfate, are generally soluble in water. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s).
from www.numerade.com
If its solubility is greater than 0.1 mol/l, we call it soluble. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. Is zinc sulfate soluble in water? Acidic salts, such as zinc sulfate, are generally soluble in water. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l.
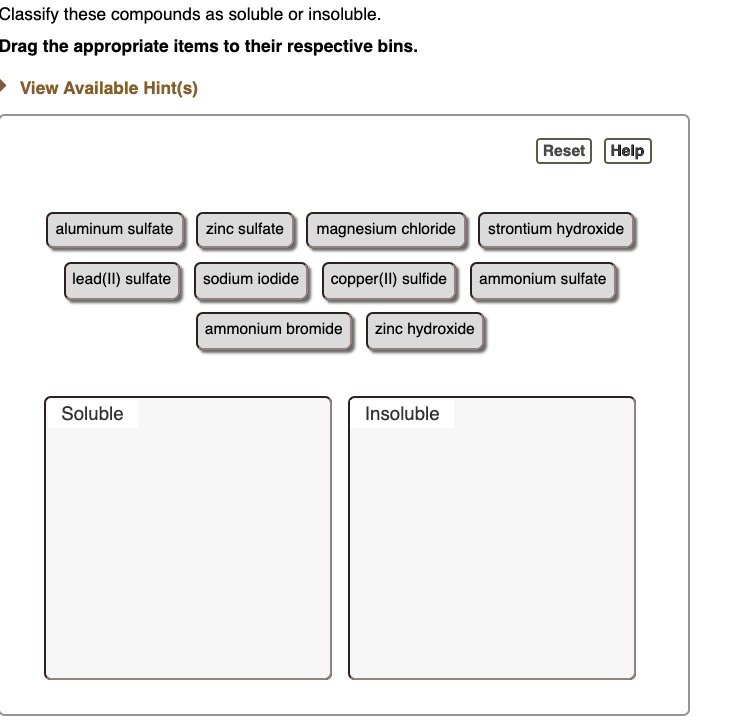
SOLVED Classify these compounds as soluble or insoluble Drag the
Zinc Sulfate Soluble Or Insoluble Acidic salts, such as zinc sulfate, are generally soluble in water. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. Acidic salts, such as zinc sulfate, are generally soluble in water. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). Is zinc sulfate soluble in water? If its solubility is greater than 0.1 mol/l, we call it soluble.
From www.youtube.com
How to Write the Formula for Zinc sulfide (ZnS) YouTube Zinc Sulfate Soluble Or Insoluble Yes, zinc sulfate is highly soluble in water, making it useful for various applications. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. Acidic salts, such as zinc sulfate, are generally soluble in water. This value is for. Zinc Sulfate Soluble Or Insoluble.
From www.bunnings.com.au
Manutec 500g Zinc Sulphate Soluble Powder Bunnings Warehouse Zinc Sulfate Soluble Or Insoluble This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. If its solubility is greater than 0.1 mol/l, we call it soluble. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. Is zinc sulfate soluble in water?. Zinc Sulfate Soluble Or Insoluble.
From www.researchgate.net
Zn 2+ solubility diagram simulated by DIASTAB. Download Scientific Zinc Sulfate Soluble Or Insoluble Yes, zinc sulfate is highly soluble in water, making it useful for various applications. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. If its solubility is greater than 0.1 mol/l, we call it soluble. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic. Zinc Sulfate Soluble Or Insoluble.
From www.hanqi.com.cn
Zinc sulfate, monohydrate ZINC HANQI INDUSTRY TRADE CO.,LIMITED Zinc Sulfate Soluble Or Insoluble Acidic salts, such as zinc sulfate, are generally soluble in water. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. If its solubility is greater than 0.1 mol/l, we call it soluble. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. Yes, zinc sulfate is highly soluble in water, making it useful. Zinc Sulfate Soluble Or Insoluble.
From shzhangguan.en.made-in-china.com
Soluble Powder Agricultural Grade Using Zinc Sulfate CAS 7733020 Zinc Sulfate Soluble Or Insoluble These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. This value is for the heptahydrate form of. Zinc Sulfate Soluble Or Insoluble.
From www.researchgate.net
Solubility diagram for zinc oxide Download Scientific Diagram Zinc Sulfate Soluble Or Insoluble This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. In general chemistry. Zinc Sulfate Soluble Or Insoluble.
From sciencenotes.org
Solubility Rules Chart and Memorization Tips Zinc Sulfate Soluble Or Insoluble In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Is zinc sulfate soluble in water? These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). Yes, zinc sulfate is highly soluble in water, making it useful for. Zinc Sulfate Soluble Or Insoluble.
From www.walmart.com
Cesco Solutions Zinc Sulfate Fertilizer Powder 99 Pure Zinc Sulfate Zinc Sulfate Soluble Or Insoluble The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. Is zinc sulfate soluble in water? This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver. Zinc Sulfate Soluble Or Insoluble.
From www.spic.in
SPIC Zinc Sulphate (Zinc Sulphate 33) [Monohydrate] Southern Zinc Sulfate Soluble Or Insoluble Acidic salts, such as zinc sulfate, are generally soluble in water. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. 133 rows we call any substance insoluble its solubility is less. Zinc Sulfate Soluble Or Insoluble.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Sulfate Soluble Or Insoluble This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. Acidic salts, such as zinc sulfate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. Yes, zinc sulfate is highly soluble in. Zinc Sulfate Soluble Or Insoluble.
From www.dreamstime.com
Soluble and Insoluble Science Experiment Stock Vector Illustration of Zinc Sulfate Soluble Or Insoluble These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). Yes, zinc sulfate is highly soluble in water, making it useful for various applications. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. The resulting solutions contain moderate concentrations of hydrogen ions. Zinc Sulfate Soluble Or Insoluble.
From www.amazon.ca
Zinc SulphateWater Soluble Zinc Sulfate Monohydrate Powder 35.5, (2 Zinc Sulfate Soluble Or Insoluble The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). This value is for the heptahydrate form. Zinc Sulfate Soluble Or Insoluble.
From www.ebay.com.au
ZINC SULPHATE Soluble Powder Zinc Sulfate High Purity Powder Analytical Zinc Sulfate Soluble Or Insoluble In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Is zinc sulfate soluble in water? 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. The solubility of zinc sulphate (znso4). Zinc Sulfate Soluble Or Insoluble.
From www.indiamart.com
Zinc Sulphate Heptahydrate, Soluble In Water, Packaging Size 25 Kg at Zinc Sulfate Soluble Or Insoluble Acidic salts, such as zinc sulfate, are generally soluble in water. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. If. Zinc Sulfate Soluble Or Insoluble.
From www.numerade.com
SOLVED Based on the solubility rules, identify whether the following Zinc Sulfate Soluble Or Insoluble Is zinc sulfate soluble in water? The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. If its solubility is greater than 0.1 mol/l, we call it soluble. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. This value is for the heptahydrate form. Zinc Sulfate Soluble Or Insoluble.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Sulfate Soluble Or Insoluble 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. Is zinc sulfate soluble in water? In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Acidic salts, such as zinc sulfate,. Zinc Sulfate Soluble Or Insoluble.
From www.shimico.com
Zinc sulfate and the methods of production Shimico blog Zinc Sulfate Soluble Or Insoluble These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. In general chemistry 1 students memorized a series of solubility rules (section. Zinc Sulfate Soluble Or Insoluble.
From www.kissanghar.pk
Solu Zinc 1kg Soluble Zinc Sulphate Heptahydrate Zinc ( Zn ) 33 Sulphur Zinc Sulfate Soluble Or Insoluble If its solubility is greater than 0.1 mol/l, we call it soluble. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. Is zinc sulfate soluble in water? Yes, zinc sulfate is highly soluble in water, making it useful for various applications. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag. Zinc Sulfate Soluble Or Insoluble.
From www.numerade.com
SOLVED Classify these compounds as soluble or insoluble Drag the Zinc Sulfate Soluble Or Insoluble In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Is zinc sulfate soluble in water? Yes, zinc sulfate is highly soluble in water, making it useful for various applications. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. 133 rows we call any substance insoluble its. Zinc Sulfate Soluble Or Insoluble.
From www.vectorstock.com
Zinc sulfate is a molecular chemical formula Vector Image Zinc Sulfate Soluble Or Insoluble The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. Is zinc sulfate soluble in water? If its solubility is greater than 0.1 mol/l, we call it soluble. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. Acidic salts, such as zinc sulfate, are generally soluble in water. This value. Zinc Sulfate Soluble Or Insoluble.
From studymind.co.uk
ᐉ Solubility Rules Insoluble & Soluble Salts Making Zinc Sulfate Soluble Or Insoluble These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. If its solubility is greater than 0.1 mol/l, we call. Zinc Sulfate Soluble Or Insoluble.
From spanish.alibaba.com
Sulfato De Zinc Monohidratado En Polvo,Soluble,Znso4.h2o Buy Sulfato Zinc Sulfate Soluble Or Insoluble The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. Acidic salts, such as zinc sulfate, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic. Zinc Sulfate Soluble Or Insoluble.
From advancedchemsys.com
Zinc Removal From Water Advanced Chemical Systems Zinc Sulfate Soluble Or Insoluble In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. If its solubility is greater than 0.1 mol/l, we call it soluble. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. The resulting. Zinc Sulfate Soluble Or Insoluble.
From www.walmart.com
Cesco Solutions Zinc Sulfate Fertilizer Powder 99 Pure Zinc Sulfate Zinc Sulfate Soluble Or Insoluble If its solubility is greater than 0.1 mol/l, we call it soluble. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o),. Zinc Sulfate Soluble Or Insoluble.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Zinc Sulfate Soluble Or Insoluble These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. This value is for the heptahydrate form of. Zinc Sulfate Soluble Or Insoluble.
From www.youtube.com
Is ZnCO3 Soluble or Insoluble in Water? YouTube Zinc Sulfate Soluble Or Insoluble Acidic salts, such as zinc sulfate, are generally soluble in water. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. If its solubility is greater than 0.1 mol/l, we call it soluble. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. 133 rows we call any substance insoluble its solubility is. Zinc Sulfate Soluble Or Insoluble.
From www.numerade.com
SOLVED Classify these compounds as soluble or insoluble. Drag the Zinc Sulfate Soluble Or Insoluble The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver sulfide (ag 2 s), and lead sulfide (pb 2 s). The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. Is zinc sulfate soluble in water? This. Zinc Sulfate Soluble Or Insoluble.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Sulfate Soluble Or Insoluble The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. If its solubility is greater than 0.1 mol/l, we call it soluble. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. These include cadmium sulfide (cds), iron. Zinc Sulfate Soluble Or Insoluble.
From socratic.org
How does zinc form an insoluble zinc hydroxide in water? Socratic Zinc Sulfate Soluble Or Insoluble The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. Is zinc sulfate soluble in water? In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. If its solubility is greater than 0.1 mol/l, we call it soluble. Acidic salts, such as zinc sulfate,. Zinc Sulfate Soluble Or Insoluble.
From www.tradeindia.com
Zinc Sulphate Powder at 10000.00 INR in Ahmedabad, Gujarat Aries Zinc Sulfate Soluble Or Insoluble 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. The solubility of zinc sulphate (znso4) in water is approximately 57.7 g/100 ml at 20°c. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound. Zinc Sulfate Soluble Or Insoluble.
From www.indiamart.com
Zinc Sulphate Monohydrate Powder 33, Soluble In Water at Rs 68/kg in Zinc Sulfate Soluble Or Insoluble Yes, zinc sulfate is highly soluble in water, making it useful for various applications. If its solubility is greater than 0.1 mol/l, we call it soluble. The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. Acidic salts, such as zinc sulfate, are generally soluble in water. This value is for the heptahydrate form. Zinc Sulfate Soluble Or Insoluble.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Sulfate Soluble Or Insoluble Is zinc sulfate soluble in water? Yes, zinc sulfate is highly soluble in water, making it useful for various applications. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. If its solubility is greater than 0.1 mol/l, we call it soluble. The solubility. Zinc Sulfate Soluble Or Insoluble.
From slideplayer.com
Unit 13 More Chemical Reactions ppt download Zinc Sulfate Soluble Or Insoluble In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. Is zinc sulfate soluble in water? The resulting solutions contain moderate concentrations of hydrogen ions and have ph's of less than 7.0. This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. 133 rows we call any substance. Zinc Sulfate Soluble Or Insoluble.
From www.walmart.com
Zinc Sulfate 220 mg 100 Count Tablets Zinc Sulfate Soluble Or Insoluble This value is for the heptahydrate form of zinc sulphate (znso4·7h2o), also. If its solubility is greater than 0.1 mol/l, we call it soluble. Yes, zinc sulfate is highly soluble in water, making it useful for various applications. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. The resulting. Zinc Sulfate Soluble Or Insoluble.
From www.alibaba.com
Zinc Sulphate Heptahydrate Znso4.7h2o Zn 21 Feed Grade Zinc Sulfate Zinc Sulfate Soluble Or Insoluble Yes, zinc sulfate is highly soluble in water, making it useful for various applications. 133 rows we call any substance insoluble its solubility is less than 0.01 mol/l. In general chemistry 1 students memorized a series of solubility rules (section 3.4.3) to predict if an ionic compound was. These include cadmium sulfide (cds), iron sulfide (fes), zinc sulfide (zns), silver. Zinc Sulfate Soluble Or Insoluble.