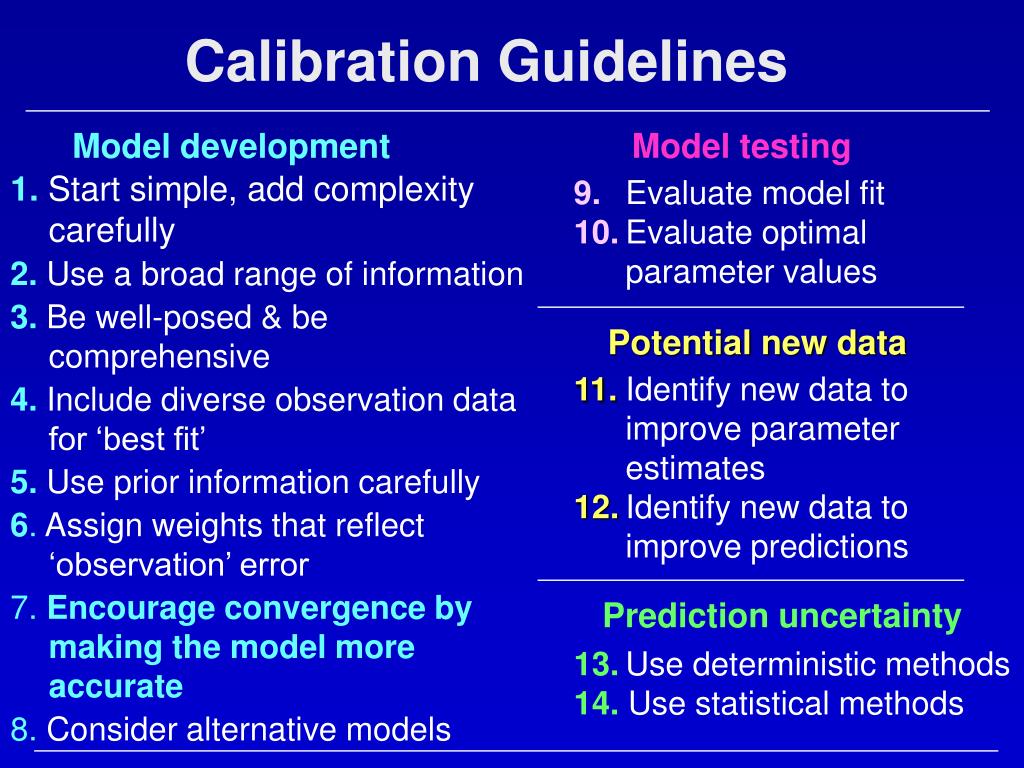
Who Guidelines For Calibration And Validation Of Equipment Slideshare . Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. It provides definitions of calibration and validation, and.
from www.slideserve.com
Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. It provides definitions of calibration and validation, and. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development.
PPT Calibration Guidelines PowerPoint Presentation, free download
Who Guidelines For Calibration And Validation Of Equipment Slideshare Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. It provides definitions of calibration and validation, and. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g.
From www.studypool.com
SOLUTION Who guidelines for calibration and validation of equipments Who Guidelines For Calibration And Validation Of Equipment Slideshare The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From pharmaguddu.com
Difference Between Validation, Calibration, and Qualification in Pharma Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideserve.com
PPT A Seminar On Validation Master Plan(VMP) & Calibration Master Who Guidelines For Calibration And Validation Of Equipment Slideshare Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Validation protocol description of the equipment to. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.scribd.com
Revised Guidelines for the Calibration of Testing Equipment of the DPWH Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideserve.com
PPT Calibration Guidelines PowerPoint Presentation, free download Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. It provides definitions of calibration and validation, and. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who). Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideshare.net
validation and calibration of HPLC Who Guidelines For Calibration And Validation Of Equipment Slideshare The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideshare.net
DATION OF EQUIPMENT ICH AND WHO GUIDELINES FOR CALIBRATION AND VALIDA… Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. It provides definitions of calibration and validation, and. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. Validation protocol description of the equipment to be used (including calibration. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.researchgate.net
(PDF) ISCEV guidelines for calibration and verification of stimuli and Who Guidelines For Calibration And Validation Of Equipment Slideshare Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines for calibration and validation. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideserve.com
PPT Qualification and Validation PowerPoint Presentation, free Who Guidelines For Calibration And Validation Of Equipment Slideshare Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideshare.net
Validation and calibration of equipment Who Guidelines For Calibration And Validation Of Equipment Slideshare It provides definitions of calibration and validation, and. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From mavink.com
Difference Between Calibration And Validation Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. Similarly, a calibration master plan. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From dokumen.tips
(PDF) Calibration Verification Guidelines and Regulations · 26 Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Similarly, a calibration master plan ensures. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.scribd.com
Equipment and Calibration Calibration Verification And Validation Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. It provides definitions of calibration and validation, and. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,.. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideshare.net
Ich & who guidelines for calibration and validation 22 PPT Who Guidelines For Calibration And Validation Of Equipment Slideshare Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. It provides definitions of calibration and validation, and. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper.. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.researchgate.net
(PDF) ICH and WHO Guideline for validation and calibration Who Guidelines For Calibration And Validation Of Equipment Slideshare Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideshare.net
DATION OF EQUIPMENT ICH AND WHO GUIDELINES FOR CALIBRATION AND VALIDA… Who Guidelines For Calibration And Validation Of Equipment Slideshare The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. It provides definitions of calibration and validation, and. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. Validation protocol description of the equipment. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.pharmaspecialists.com
Difference Between Calibration and Validation Who Guidelines For Calibration And Validation Of Equipment Slideshare It provides definitions of calibration and validation, and. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideshare.net
Validation, scope of validation, URS , WHO GUIDELINES FOR VALIDATION Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. Analytical procedure validation forms a part of the. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From xiltrixusa.com
Calibration and Validation in the Lab Who Guidelines For Calibration And Validation Of Equipment Slideshare Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.studypool.com
SOLUTION Who guidelines for calibration and validation of equipments Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. Validation protocol description of the equipment. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideshare.net
validation and calibration of HPLC Who Guidelines For Calibration And Validation Of Equipment Slideshare Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. It provides definitions of calibration and validation, and. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From pharmdguru.com
3. VALIDATION METHODS QUALITY OF EQUIPMENT, VALIDATION OF EQUIPMENT Who Guidelines For Calibration And Validation Of Equipment Slideshare Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. It provides definitions of calibration and validation, and. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. The purpose of this discussion is an attempt to see the various aspects of validation that. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From studylib.net
CalibrationValidation Who Guidelines For Calibration And Validation Of Equipment Slideshare Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. The purpose of this discussion is an attempt. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideserve.com
PPT Calibration and Electrical Safety of Medical Equipment PowerPoint Who Guidelines For Calibration And Validation Of Equipment Slideshare The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. It provides. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From mpl.loesungsfabrik.de
Calibration, qualification and validation in the lab what's that? Who Guidelines For Calibration And Validation Of Equipment Slideshare Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From joinkvzks.blob.core.windows.net
Calibration Of Instruments Ppt at Doug Meyer blog Who Guidelines For Calibration And Validation Of Equipment Slideshare Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. It provides definitions of calibration and validation, and. The purpose of this discussion is an attempt to see the various aspects of validation that. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.youtube.com
Calibration and validation YouTube Who Guidelines For Calibration And Validation Of Equipment Slideshare Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. It provides definitions of calibration and validation, and. The document discusses guidelines for calibration and validation of equipment as outlined. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.scribd.com
HVAC System Qualification Protocol (Validation) Pharmaceutical Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. The document discusses guidelines for calibration and validation of equipment as outlined by ich. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.slideshare.net
validation and calibration of HPLC Who Guidelines For Calibration And Validation Of Equipment Slideshare Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.youtube.com
Introduction to calibration and validation YouTube Who Guidelines For Calibration And Validation Of Equipment Slideshare Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. It provides definitions of calibration and validation,. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.researchgate.net
Schematic diagram of typical model calibration and validation steps Who Guidelines For Calibration And Validation Of Equipment Slideshare The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. It provides definitions of calibration and validation, and. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. Validation protocol description of the equipment to be used (including calibration before. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From exysasktf.blob.core.windows.net
Calibration And Validation As Per Ich at Daryl White blog Who Guidelines For Calibration And Validation Of Equipment Slideshare Validation protocol description of the equipment to be used (including calibration before and after validation) sops to be followed (e.g. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. The document discusses guidelines from the international conference on harmonization (ich) and the world health organization (who) for validating equipment used. The purpose of. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From www.presentationeze.com
Equipment Validation Facility Qualification Material Who Guidelines For Calibration And Validation Of Equipment Slideshare Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. It provides. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From calibrationawareness.com
How to Differentiate Calibration, Verification, and Validation Who Guidelines For Calibration And Validation Of Equipment Slideshare It provides definitions of calibration and validation, and. Calibration and verifi cation 7.1 calibration and verifi cation of equipment, instruments and other devices, as applicable,. Analytical procedure validation forms a part of the analytical procedure lifecycle, as described within ich q14 analytical procedure development. Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper.. Who Guidelines For Calibration And Validation Of Equipment Slideshare.
From dokumen.tips
(PDF) Guidelines the Calibration Evaluation of Systems for for the Who Guidelines For Calibration And Validation Of Equipment Slideshare Similarly, a calibration master plan ensures equipment is routinely calibrated against reference standards to ensure proper. The document discusses guidelines for calibration and validation of equipment as outlined by ich and who. The purpose of this discussion is an attempt to see the various aspects of validation that includes components of water treatment systems, qualification of equipment,. The document discusses. Who Guidelines For Calibration And Validation Of Equipment Slideshare.