Does Bases React With Aluminium . Last updated at april 16, 2024 by teachoo. But others, called amphoteric metals, may form salts with them. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. But all metals do not react with bases to produce hydrogen gas. Most metals will not react with bases. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Some metals react with bases to produce salt and hydrogen gas. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. You can think of lone pairs on hydroxide ions as forming dative covalent.
from sciencewithmrsb.weebly.com
Some metals react with bases to produce salt and hydrogen gas. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. Last updated at april 16, 2024 by teachoo. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. You can think of lone pairs on hydroxide ions as forming dative covalent. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. But others, called amphoteric metals, may form salts with them. But all metals do not react with bases to produce hydrogen gas. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas.
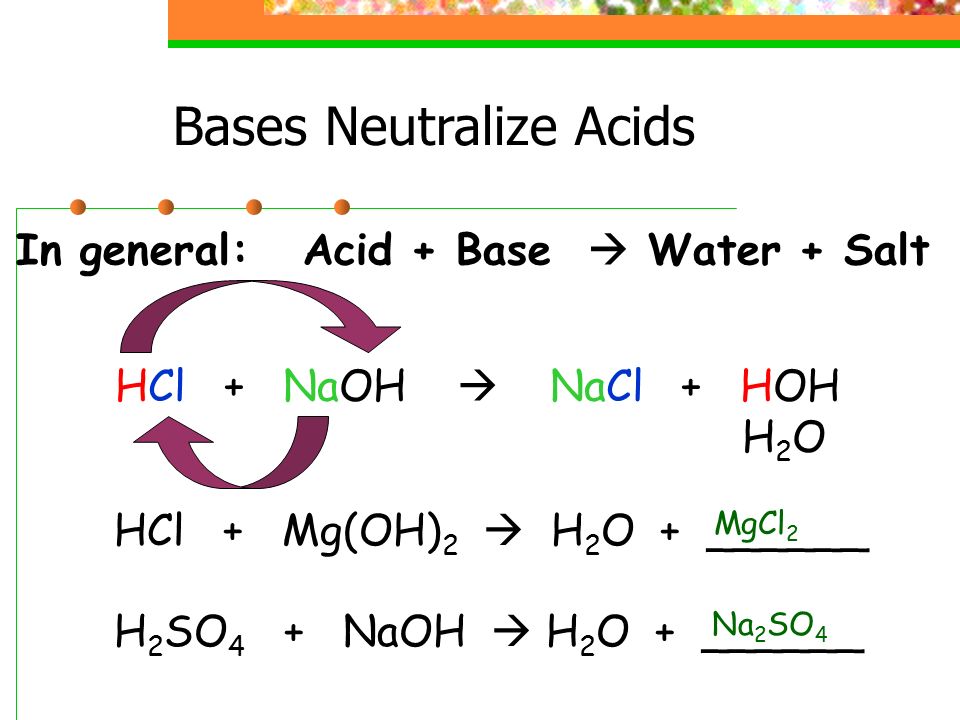
Acids and Bases Science with Mrs Beggs
Does Bases React With Aluminium But all metals do not react with bases to produce hydrogen gas. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. But all metals do not react with bases to produce hydrogen gas. Most metals will not react with bases. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Last updated at april 16, 2024 by teachoo. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. But others, called amphoteric metals, may form salts with them. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Some metals react with bases to produce salt and hydrogen gas. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. You can think of lone pairs on hydroxide ions as forming dative covalent. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen.
From blueagents.weebly.com
Last Reaction And Water Bases blueagents Does Bases React With Aluminium Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. But others, called amphoteric metals, may form salts with them. Some metals react with bases to produce salt and hydrogen gas. But all metals do not react with bases to produce. Does Bases React With Aluminium.
From www.youtube.com
How do Acids and Bases React with Metals in Hindi for Class 10 YouTube Does Bases React With Aluminium But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. But others, called amphoteric metals, may form salts with them. Last updated at april 16, 2024 by teachoo. Most metals will not react with bases. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Aluminum. Does Bases React With Aluminium.
From byjus.com
does aluminium react with water Does Bases React With Aluminium Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Most metals will not react with bases. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. Last updated at april 16, 2024 by teachoo. Aluminium oxide contains oxide ions and so reacts with acids in the same way as. Does Bases React With Aluminium.
From www.alamy.com
Thin layer of aluminium oxide makes the appearance of aluminium metal Does Bases React With Aluminium But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. But others, called amphoteric metals, may form salts with them. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Bases also react with certain. Does Bases React With Aluminium.
From brainly.in
how do acids and bases react with metals Brainly.in Does Bases React With Aluminium Some metals react with bases to produce salt and hydrogen gas. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. But aluminium. Does Bases React With Aluminium.
From www.youtube.com
What happens when Sodium Hydroxide and Aluminium Reacts Does Bases React With Aluminium Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Some metals react with bases to produce salt and hydrogen gas. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. Most metals. Does Bases React With Aluminium.
From blog.thepipingmart.com
Does Aluminium React with Water? Does Bases React With Aluminium But all metals do not react with bases to produce hydrogen gas. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Most metals will not react with bases. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Metals like zinc (zn) and aluminium (al) react. Does Bases React With Aluminium.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts Does Bases React With Aluminium Some metals react with bases to produce salt and hydrogen gas. You can think of lone pairs on hydroxide ions as forming dative covalent. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Metals like zinc. Does Bases React With Aluminium.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Does Bases React With Aluminium Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Last updated at april 16, 2024 by teachoo. But others, called amphoteric metals, may form salts with them. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. But. Does Bases React With Aluminium.
From albertojaslyn.blogspot.com
Acids React With Bases To Form Does Bases React With Aluminium Some metals react with bases to produce salt and hydrogen gas. But all metals do not react with bases to produce hydrogen gas. Most metals will not react with bases. But others, called amphoteric metals, may form salts with them. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. Bases also react with certain. Does Bases React With Aluminium.
From www.doubtnut.com
Acetylene and ethylene react with alkaline KMnO(4) to give Does Bases React With Aluminium But all metals do not react with bases to produce hydrogen gas. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Last updated at april 16, 2024. Does Bases React With Aluminium.
From www.nagwa.com
Question Video Recalling the Type of Oxide that Does Not React with Does Bases React With Aluminium Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Some metals react with bases to produce salt and hydrogen gas. You can think of lone pairs on hydroxide ions as forming dative covalent. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. But all metals do not react with bases to. Does Bases React With Aluminium.
From stock.adobe.com
Thin layer of aluminium oxide makes the appearance of aluminium metal Does Bases React With Aluminium But others, called amphoteric metals, may form salts with them. Last updated at april 16, 2024 by teachoo. Most metals will not react with bases. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Some metals react with bases to produce salt and hydrogen gas. You can think of lone pairs on hydroxide ions as. Does Bases React With Aluminium.
From slideplayer.com
Acids and Bases Acids Bases Sour Taste React with metal to form H2 Does Bases React With Aluminium Last updated at april 16, 2024 by teachoo. You can think of lone pairs on hydroxide ions as forming dative covalent. Most metals will not react with bases. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. But all metals do not react with bases to produce hydrogen gas. Bases also react with certain. Does Bases React With Aluminium.
From edurev.in
Why do all metals do not react with bases ? EduRev Class 10 Question Does Bases React With Aluminium You can think of lone pairs on hydroxide ions as forming dative covalent. But all metals do not react with bases to produce hydrogen gas. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. Most metals will not react with bases. Some metals react with bases to produce salt and hydrogen gas. Last. Does Bases React With Aluminium.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Does Bases React With Aluminium Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. You can think of lone pairs on hydroxide ions as forming dative covalent. But all metals do. Does Bases React With Aluminium.
From slideplayer.com
Acids and Bases. ppt download Does Bases React With Aluminium Most metals will not react with bases. You can think of lone pairs on hydroxide ions as forming dative covalent. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Some metals react with bases to produce. Does Bases React With Aluminium.
From www.youtube.com
How bases react with non metallic oxides CBSE Class 10 Chemistry Notes Does Bases React With Aluminium But others, called amphoteric metals, may form salts with them. Last updated at april 16, 2024 by teachoo. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Some metals react with bases to produce salt and hydrogen gas. But all metals do not react with bases to produce hydrogen gas. You can think of lone pairs. Does Bases React With Aluminium.
From www.youtube.com
Type of Reaction for Al + HCl = AlCl3 + H2 YouTube Does Bases React With Aluminium But others, called amphoteric metals, may form salts with them. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. But all metals do not react with bases to produce hydrogen gas. Last updated at april 16, 2024 by teachoo. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas.. Does Bases React With Aluminium.
From www.numerade.com
SOLVED Which statement about bases is true? Bases increase the Does Bases React With Aluminium Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. But all metals do not react with bases to produce hydrogen gas. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Last updated at april 16, 2024 by teachoo. Aluminum oxide also displays acidic properties, as shown. Does Bases React With Aluminium.
From www.teachoo.com
What do all Acids and Bases have in common? (with 5+ Points) Teachoo Does Bases React With Aluminium Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Last updated at april 16, 2024 by teachoo. But all metals do not react with bases to produce hydrogen gas. Aluminium oxide contains oxide ions and so reacts with acids in the. Does Bases React With Aluminium.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UW Does Bases React With Aluminium Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. But aluminium oxide. Does Bases React With Aluminium.
From www.youtube.com
How bases react with metals CBSE Class 10 Chemistry Notes YouTube Does Bases React With Aluminium Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Most metals will not react with bases. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. Some metals react with bases to produce salt and hydrogen gas. Metals like zinc (zn) and aluminium (al) react. Does Bases React With Aluminium.
From byjus.com
The balanced net ionic equation for the reaction of aluminium sulphate Does Bases React With Aluminium But all metals do not react with bases to produce hydrogen gas. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Most metals will not react with bases. Some metals react with bases to produce salt and hydrogen gas. Aluminum oxide also displays acidic properties, as shown in its reactions. Does Bases React With Aluminium.
From www.teachoo.com
How do Acids and Bases react with Metals? [with Examples] Teachoo Does Bases React With Aluminium But all metals do not react with bases to produce hydrogen gas. Last updated at april 16, 2024 by teachoo. You can think of lone pairs on hydroxide ions as forming dative covalent. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. Bases also react with certain metals, like zinc or aluminum, to. Does Bases React With Aluminium.
From www.youtube.com
How acids react with metallic oxides CBSE Class 10 Chemistry Notes Does Bases React With Aluminium But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. Some metals react with bases to produce salt and hydrogen gas. But all metals do not react with bases to produce hydrogen gas. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Last updated at. Does Bases React With Aluminium.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts Does Bases React With Aluminium But all metals do not react with bases to produce hydrogen gas. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Most metals will not react with bases. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Aluminium oxide contains oxide ions and so reacts with acids in the same. Does Bases React With Aluminium.
From www.chemicals.co.uk
What is the Difference Between an Acid and a Base? The Chemistry Blog Does Bases React With Aluminium Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. But others, called amphoteric metals, may form salts with them. Most metals will not react with bases. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. Aluminum oxide also displays acidic properties, as shown in its. Does Bases React With Aluminium.
From www.youtube.com
Reaction Of bases With NonMetal Oxides 10 YouTube Does Bases React With Aluminium Last updated at april 16, 2024 by teachoo. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Most metals will not. Does Bases React With Aluminium.
From www.istockphoto.com
Acids React With Bases To Produce Salt And Water Stock Illustration Does Bases React With Aluminium But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. But others, called amphoteric metals, may form salts with them. But all metals do not react with bases to produce hydrogen gas. Some metals react with bases to produce. Does Bases React With Aluminium.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Does Bases React With Aluminium Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. But all metals do not react with bases to produce hydrogen gas. Most metals will not react with bases. Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Bases also react with certain metals, like zinc or. Does Bases React With Aluminium.
From www.scribd.com
How Do Bases React With Metals PDF Does Bases React With Aluminium But all metals do not react with bases to produce hydrogen gas. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Last updated at april 16, 2024 by teachoo. You can think of lone. Does Bases React With Aluminium.
From brainly.in
how do acid and base react with metal Brainly.in Does Bases React With Aluminium But all metals do not react with bases to produce hydrogen gas. Last updated at april 16, 2024 by teachoo. Some metals react with bases to produce salt and hydrogen gas. But aluminium oxide reacts with bases like sodium hydroxide solution to form complex aluminate ions. Aluminum hydroxide is amphoteric which means that it can react with acids or bases.. Does Bases React With Aluminium.
From trivaldypiping.blogspot.com
Do Bases React With Metals To Produce Hydrogen Gas Does Bases React With Aluminium Metals like zinc (zn) and aluminium (al) react with bases to produce hydrogen. Last updated at april 16, 2024 by teachoo. Some metals react with bases to produce salt and hydrogen gas. Aluminum oxide also displays acidic properties, as shown in its reactions with bases such as sodium. You can think of lone pairs on hydroxide ions as forming dative. Does Bases React With Aluminium.
From www.numerade.com
Metal oxides can react with water to form bases. Does Bases React With Aluminium Aluminium oxide contains oxide ions and so reacts with acids in the same way as sodium or magnesium oxides. Bases also react with certain metals, like zinc or aluminum, to produce hydrogen gas. But others, called amphoteric metals, may form salts with them. Aluminum hydroxide is amphoteric which means that it can react with acids or bases. Some metals react. Does Bases React With Aluminium.