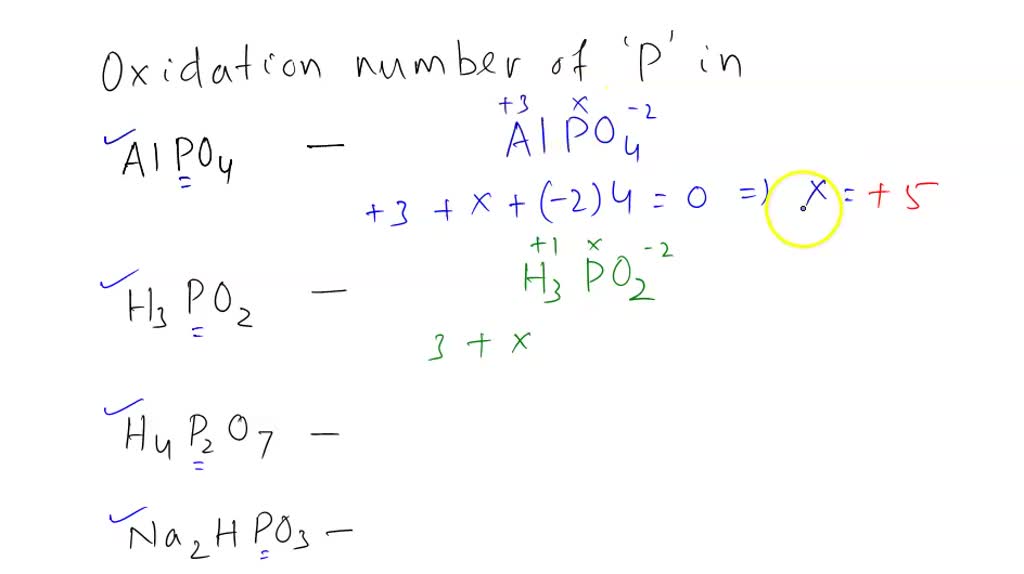Jeopardylabs Oxidation State . 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. What is the oxidation state of. What is an oxidation state? The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. How do you determine the value of an element's oxidation state? From its position in the periodic table and/or the other element(s) present in the formula the. What are the rules for determining oxidation states? What is an elemental state? The oxidation number of any uncombined element is zero. How do you determine the value of an element’s oxidation state? The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been.
from www.numerade.com
What are the rules for determining oxidation states? What is the oxidation state of. How do you determine the value of an element’s oxidation state? The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. From its position in the periodic table and/or the other element(s) present in the formula the. How do you determine the value of an element's oxidation state? What is an oxidation state? What is an elemental state? 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond.
SOLVED I. Determine the oxidation number for phophorus in each of the
Jeopardylabs Oxidation State What is an elemental state? What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. What is an elemental state? What is an oxidation state? 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. From its position in the periodic table and/or the other element(s) present in the formula the. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What are the rules for determining oxidation states? How do you determine the value of an element’s oxidation state? Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been. The oxidation number of any uncombined element is zero. How do you determine the value of an element's oxidation state? What is the oxidation state of.
From askfilo.com
Consider the change in oxidation state of Bromine corresponding to differ.. Jeopardylabs Oxidation State What is an elemental state? From its position in the periodic table and/or the other element(s) present in the formula the. How do you determine the value of an element’s oxidation state? The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. The oxidation state of an atom. Jeopardylabs Oxidation State.
From www.numerade.com
SOLVED I. Determine the oxidation number for phophorus in each of the Jeopardylabs Oxidation State What is an oxidation state? The oxidation number of any uncombined element is zero. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. What is an elemental state? How do you determine the value of an element's oxidation state? What is the oxidation state. Jeopardylabs Oxidation State.
From www.numerade.com
SOLVED . Complete the rules for assigning oxidation numbers to atoms Jeopardylabs Oxidation State Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been. The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. What are the rules for determining oxidation states? 119 rows the oxidation state tells how. Jeopardylabs Oxidation State.
From www.doubtnut.com
Give the oxidation state, dorbitals occupation and coordination numbe Jeopardylabs Oxidation State What is the oxidation state of. The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. What is an elemental state? What is an. Jeopardylabs Oxidation State.
From edurev.in
Find oxidation number of P in Pb3O4,C in C3O2 and Br in Br3O8 Jeopardylabs Oxidation State What is the oxidation state of. What is an oxidation state? The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been. The oxidation state of. Jeopardylabs Oxidation State.
From readchemistry.com
Oxidation states of Alcohols and Related Functional Groups Read Chemistry Jeopardylabs Oxidation State The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. What is the oxidation state of. The oxidation number of any uncombined element is zero. What is an elemental state? 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive. Jeopardylabs Oxidation State.
From www.numerade.com
SOLVED Part A) The oxidation state of oxygen in sodium peroxide, Na2O2 Jeopardylabs Oxidation State Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been. The oxidation number of any uncombined element is zero. The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. 119 rows the oxidation state tells. Jeopardylabs Oxidation State.
From achs-prod.acs.org
OxidationStateDependent Binding Properties of the Active Site in a Mo Jeopardylabs Oxidation State The oxidation number of any uncombined element is zero. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. How do you determine the value of an element’s oxidation state? How do you determine the value of an element's oxidation state? From its position in. Jeopardylabs Oxidation State.
From www.numerade.com
SOLVED What is the oxidation number of nitrogen in nitric acid HNO3?a Jeopardylabs Oxidation State The oxidation number of any uncombined element is zero. What is an oxidation state? From its position in the periodic table and/or the other element(s) present in the formula the. The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. How do you determine the value of an. Jeopardylabs Oxidation State.
From www.youtube.com
How to find the Oxidation Number for P in P4O10 (Phosphorus Pentoxide Jeopardylabs Oxidation State What is the oxidation state of. How do you determine the value of an element’s oxidation state? What is an oxidation state? The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number). Jeopardylabs Oxidation State.
From solvedlib.com
What Is the oxidation state of iodine in potassium io… SolvedLib Jeopardylabs Oxidation State What are the rules for determining oxidation states? The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What is the oxidation state of. How do you determine the value of an element’s oxidation state? Define oxidation and reduction in terms of electron loss and gain., if. Jeopardylabs Oxidation State.
From www.researchgate.net
(A) Oxidation states of sulfur (S) in proteins from thiol (2) to Jeopardylabs Oxidation State What are the rules for determining oxidation states? What is an oxidation state? How do you determine the value of an element’s oxidation state? What is the oxidation state of. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. How do you determine the. Jeopardylabs Oxidation State.
From www.youtube.com
The oxidation number of S in H2S2O8 is Redox Master Series Master Jeopardylabs Oxidation State What are the rules for determining oxidation states? The oxidation number of any uncombined element is zero. The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing. Jeopardylabs Oxidation State.
From www.youtube.com
Oxidation Number or Oxidation State Class 9 Science Term 2 Unit 13 Jeopardylabs Oxidation State What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been. What is an elemental state? How do you determine the value of. Jeopardylabs Oxidation State.
From www.animalia-life.club
Oxidation State Of N Jeopardylabs Oxidation State What is an oxidation state? How do you determine the value of an element’s oxidation state? The oxidation number of any uncombined element is zero. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. From its position in the periodic table and/or the other element(s). Jeopardylabs Oxidation State.
From mydigitalkemistry.com
Difference between Oxidation and Reduction, Oxidizing agent and Jeopardylabs Oxidation State The oxidation number of any uncombined element is zero. How do you determine the value of an element's oxidation state? From its position in the periodic table and/or the other element(s) present in the formula the. How do you determine the value of an element’s oxidation state? What is an oxidation state? The oxidation state of an atom is equal. Jeopardylabs Oxidation State.
From www.wou.edu
CH150 Chapter 5 Chemical Reactions Chemistry Jeopardylabs Oxidation State How do you determine the value of an element's oxidation state? 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. From its. Jeopardylabs Oxidation State.
From www.mdpi.com
Reactions Free FullText Hydrothermal Synthesis of Vanadium Oxide Jeopardylabs Oxidation State The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What is the oxidation state of. From its position in the periodic table and/or the other element(s) present in the formula the. The oxidation number of any uncombined element is zero. Define oxidation and reduction in terms. Jeopardylabs Oxidation State.
From www.numerade.com
SOLVED Calculate the oxidation state of carbon in CH3OH Jeopardylabs Oxidation State The oxidation number of any uncombined element is zero. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. The process by. Jeopardylabs Oxidation State.
From quizlet.com
What is the oxidation number of \ce{Br} in \ce{Br3O8}? Quizlet Jeopardylabs Oxidation State The oxidation number of any uncombined element is zero. Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been. 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. What are the rules. Jeopardylabs Oxidation State.
From www.chemistrylearner.com
Free Printable Oxidation Numbers Worksheets Jeopardylabs Oxidation State From its position in the periodic table and/or the other element(s) present in the formula the. What are the rules for determining oxidation states? What is the oxidation state of. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What is the definition of oxidation and. Jeopardylabs Oxidation State.
From www.doubtnut.com
The oxidation state of osmium (Os) in OsO4 is Jeopardylabs Oxidation State The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. From its position in the periodic table and/or the other element(s) present in the formula the. Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element. Jeopardylabs Oxidation State.
From www.doubtnut.com
The oxidation state of Mo in its species [Mo(2)O(4)(C(2)H( Jeopardylabs Oxidation State What is an elemental state? How do you determine the value of an element’s oxidation state? What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. What is an oxidation state? How do you determine the value of an element's oxidation state? From its position. Jeopardylabs Oxidation State.
From www.researchgate.net
DFT calculations a ΔGH* as a function of oxidation state of Os SACs. b Jeopardylabs Oxidation State What is an oxidation state? From its position in the periodic table and/or the other element(s) present in the formula the. The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. How do you determine the value of an element's oxidation state? How do you determine the value. Jeopardylabs Oxidation State.
From www.toppr.com
What is the hybrid state and oxidation state of sulphur in Caro's acid? Jeopardylabs Oxidation State The oxidation number of any uncombined element is zero. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What is an oxidation state? How do you determine the value of an element's oxidation state? What are the rules for determining oxidation states? What is an elemental. Jeopardylabs Oxidation State.
From truyenhinhcapsongthu.net
72 Balance MnO4 +H2C2O4 >Mn2+ +Co2+h2o By Oxidation Byju's Jeopardylabs Oxidation State What is the oxidation state of. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. From its position in the periodic table and/or the other element(s) present in the formula the. What is an oxidation state? The process by which a substance loses one. Jeopardylabs Oxidation State.
From askfilo.com
The Oxidation state of nickel in Ni(CO)4 is Filo Jeopardylabs Oxidation State What is an oxidation state? 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. What is an elemental state? What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. How. Jeopardylabs Oxidation State.
From www.numerade.com
SOLVED '4MnO4 3S,03+ 2OH AMnOz 6S032+ Hzo In the above reaction, the Jeopardylabs Oxidation State The oxidation number of any uncombined element is zero. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. From its position in the periodic table and/or the other element(s) present in the formula the. What is an elemental state? The oxidation state of an. Jeopardylabs Oxidation State.
From www.numerade.com
What is the oxidation number of carbon in C2H4O2? Jeopardylabs Oxidation State What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how do you. How do you determine the value of an element's oxidation state? The oxidation number of any uncombined element is zero. Define oxidation and reduction in terms of electron loss and gain., if the oxidation number. Jeopardylabs Oxidation State.
From nrochemistry.com
ParikhDoering Oxidation Jeopardylabs Oxidation State 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. The process by which a substance loses one or more electrons, this reaction involves both oxidation and reduction, oxidation is loss of. What are the rules for determining oxidation states? What is the definition of oxidation. Jeopardylabs Oxidation State.
From pubs.acs.org
Coordination Geometry and Oxidation State Requirements of Corner Jeopardylabs Oxidation State The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What is an elemental state? Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been. The process by which a substance loses one or. Jeopardylabs Oxidation State.
From askfilo.com
The oxidation states of P atom in POCl3 ,H2 PO3 and H4 P2 O6 , respectiv.. Jeopardylabs Oxidation State How do you determine the value of an element’s oxidation state? The oxidation number of any uncombined element is zero. What are the rules for determining oxidation states? 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. From its position in the periodic table and/or. Jeopardylabs Oxidation State.
From pubs.acs.org
Mechanistic Insights into the Oxidative and Reductive Quenching Cycles Jeopardylabs Oxidation State What is an elemental state? How do you determine the value of an element's oxidation state? Define oxidation and reduction in terms of electron loss and gain., if the oxidation number of an element increases has the element been. The oxidation state of an atom is equal to the total number of electrons which have been removed from an element. Jeopardylabs Oxidation State.
From www.youtube.com
Oxidation state vs Oxidation number Oxi.State and Oxi.number Jeopardylabs Oxidation State What are the rules for determining oxidation states? The oxidation state of an atom is equal to the total number of electrons which have been removed from an element (producing a. What is the oxidation state of. What is the definition of oxidation and reduction?, give an example of a redox reaction and identify the oxidizing and reducing agents., how. Jeopardylabs Oxidation State.
From docslib.org
Oxidation States of Carbon Oxidation and Reduction in Biology DocsLib Jeopardylabs Oxidation State What is an oxidation state? 119 rows the oxidation state tells how many valence electrons an atom accepts (negative number) or donates (positive number) to form a chemical bond. How do you determine the value of an element’s oxidation state? The oxidation state of an atom is equal to the total number of electrons which have been removed from an. Jeopardylabs Oxidation State.