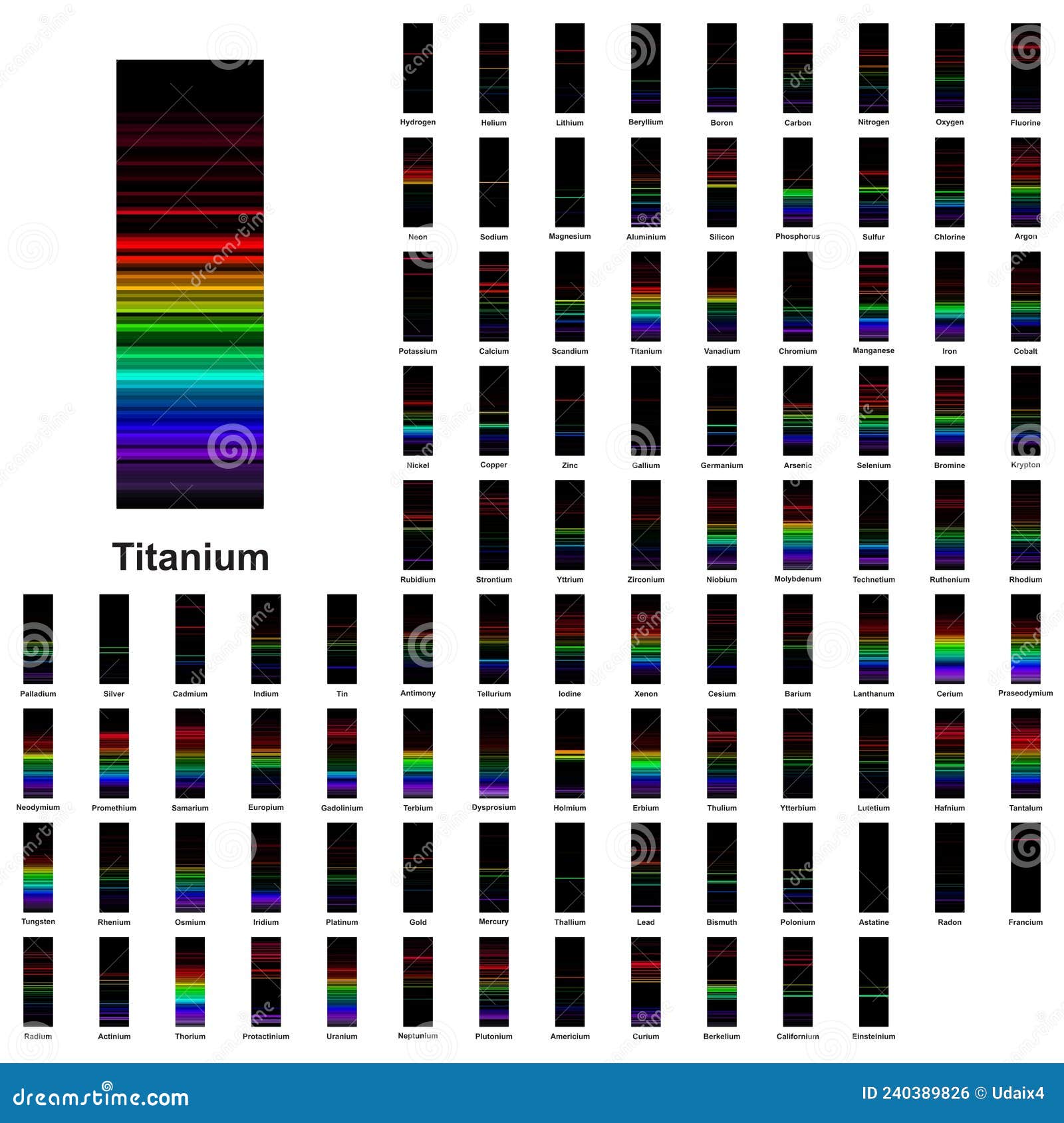
Emission Spectra Identify Elements . And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? This means they can be used to identify. The emission spectra of various atoms. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The set of individual colors emitted by an element is called its spectrum. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Continuous spectra are produced by electrons being shared between. Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. This means that line spectra can be used to identify elements. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat.
from www.dreamstime.com
Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? This means they can be used to identify. The emission spectra of various atoms. The set of individual colors emitted by an element is called its spectrum. Continuous spectra are produced by electrons being shared between. Each element has its own unique absorption spectra, just like it has a unique emission spectra. This means that line spectra can be used to identify elements. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat.
Elements Emission Spectrum List Lines Visible Light Spectra Stock
Emission Spectra Identify Elements And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? The emission spectra of various atoms. Continuous spectra are produced by electrons being shared between. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? Each element has its own unique absorption spectra, just like it has a unique emission spectra. This means that line spectra can be used to identify elements. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. This means they can be used to identify. The set of individual colors emitted by an element is called its spectrum.
From pressbooks.bccampus.ca
5.3 Spectroscopy in Astronomy Douglas College Astronomy 1105 Emission Spectra Identify Elements Continuous spectra are produced by electrons being shared between. Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. And what can explain the actual wavelength values observed in the hydrogen line spectrum and. Emission Spectra Identify Elements.
From mavink.com
Absorption Spectrum Of Hydrogen Emission Spectra Identify Elements Continuous spectra are produced by electrons being shared between. The set of individual colors emitted by an element is called its spectrum. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. This means. Emission Spectra Identify Elements.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Emission Spectra Identify Elements This means that line spectra can be used to identify elements. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The emission spectra of various atoms. And what can explain the actual wavelength values observed in the. Emission Spectra Identify Elements.
From www.dreamstime.com
Elements Emission Spectrum List Lines Visible Light Spectra Stock Emission Spectra Identify Elements Each element has its own unique absorption spectra, just like it has a unique emission spectra. The set of individual colors emitted by an element is called its spectrum. Continuous spectra are produced by electrons being shared between. This means that line spectra can be used to identify elements. The emission spectra of various atoms. And what can explain the. Emission Spectra Identify Elements.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Emission Spectra Identify Elements The emission spectra of various atoms. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. This means they can be used to identify. The set of individual colors emitted by an element is called its spectrum. Continuous spectra are produced by electrons being shared between. This means that line spectra can be used. Emission Spectra Identify Elements.
From www.pinterest.com
line spectra chart If the emission lines of the chemical elements Emission Spectra Identify Elements Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. Continuous spectra are produced by electrons being shared between. This means they can be used to identify. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to. Emission Spectra Identify Elements.
From spiff.rit.edu
Using diffraction gratings to identify elements Emission Spectra Identify Elements This means that line spectra can be used to identify elements. Continuous spectra are produced by electrons being shared between. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? The set of individual. Emission Spectra Identify Elements.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectra Identify Elements This means they can be used to identify. This means that line spectra can be used to identify elements. The set of individual colors emitted by an element is called its spectrum. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Any given element therefore has both a characteristic emission spectrum and a. Emission Spectra Identify Elements.
From laderarctic.weebly.com
Emission spectra laderarctic Emission Spectra Identify Elements This means they can be used to identify. This means that line spectra can be used to identify elements. Continuous spectra are produced by electrons being shared between. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the. Emission Spectra Identify Elements.
From www.reddit.com
Emission Spectra of the Elements in Periodic Table Format r/chemistry Emission Spectra Identify Elements Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. Continuous spectra are produced by electrons being shared between. This means they can be used to identify. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? Each element has. Emission Spectra Identify Elements.
From www.pinterest.ca
Pin de Sandra Pedro em Physics is awesome Astrofísica, Quimica geral Emission Spectra Identify Elements The set of individual colors emitted by an element is called its spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. This means that line spectra can be used to identify elements. This means they can be used to identify. Each element has its. Emission Spectra Identify Elements.
From www.sliderbase.com
Emission spectra Presentation Physics Emission Spectra Identify Elements This means they can be used to identify. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? The set of individual colors emitted by an element is called its spectrum. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The emission spectra. Emission Spectra Identify Elements.
From study.com
Quiz & Worksheet Atomic Spectra Characteristics & Types Emission Spectra Identify Elements And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. This means they can be used to identify. Since the spectrum of each element is unique,. Emission Spectra Identify Elements.
From www.resonancescience.org
What is Resonance and Why is it so Important? Emission Spectra Identify Elements The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. This means that line spectra can be used to identify elements. And what can explain the. Emission Spectra Identify Elements.
From www.esa.int
ESA Absorption and emission spectra of various elements Emission Spectra Identify Elements Continuous spectra are produced by electrons being shared between. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? This means that line spectra can be used to identify elements. The set of individual colors emitted by an element is called its spectrum. Since the spectrum of each element is. Emission Spectra Identify Elements.
From rightmetal.weebly.com
Atomic emission spectrum chemistry definition rightmetal Emission Spectra Identify Elements And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? This means they can be used to identify. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. Any given element therefore has both a characteristic. Emission Spectra Identify Elements.
From brokeasshome.com
periodic table emission spectra Emission Spectra Identify Elements Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. The emission spectra of various atoms. And what can explain the actual wavelength values observed in the hydrogen line spectrum. Emission Spectra Identify Elements.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Identify Elements Continuous spectra are produced by electrons being shared between. The set of individual colors emitted by an element is called its spectrum. Each element has its own unique absorption spectra, just like it has a unique emission spectra. This means they can be used to identify. And what can explain the actual wavelength values observed in the hydrogen line spectrum. Emission Spectra Identify Elements.
From www.visionlearning.com
Atomic Theory II Chemistry Visionlearning Emission Spectra Identify Elements The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. This means they can be used to identify. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. This means that line spectra can be used to identify elements. The. Emission Spectra Identify Elements.
From www.nagwa.com
Question Video Identifying the Emission Spectrum Corresponding to an Emission Spectra Identify Elements Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. Each element has its own unique absorption spectra, just like it has a unique emission spectra. This means that line spectra can be used to identify elements. The set of individual colors emitted by an element is called its spectrum.. Emission Spectra Identify Elements.
From www.savemyexams.com
Emission & Absorption Spectrum HL IB Physics Revision Notes 2025 Emission Spectra Identify Elements The set of individual colors emitted by an element is called its spectrum. The emission spectra of various atoms. This means that line spectra can be used to identify elements. Each element has its own unique absorption spectra, just like it has a unique emission spectra. The emission spectrum (or line spectrum) of a chemical element is the unique pattern. Emission Spectra Identify Elements.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Emission Spectra Identify Elements Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat.. Emission Spectra Identify Elements.
From ucscphysicsdemo.sites.ucsc.edu
Linear Spectra UCSC Physics Demonstration Room Emission Spectra Identify Elements Each element has its own unique absorption spectra, just like it has a unique emission spectra. This means they can be used to identify. Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. The set of individual colors emitted by an element is called its spectrum. The emission spectrum. Emission Spectra Identify Elements.
From webmis.highland.cc.il.us
Atomic Spectra and Models of the Atom Emission Spectra Identify Elements Each element has its own unique absorption spectra, just like it has a unique emission spectra. Continuous spectra are produced by electrons being shared between. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? This means they can be used to identify. Any given element therefore has both a. Emission Spectra Identify Elements.
From brokeasshome.com
periodic table emission spectra Emission Spectra Identify Elements The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. The emission spectra of various atoms. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? Any given element therefore has both a characteristic emission spectrum. Emission Spectra Identify Elements.
From www.thoughtco.com
What Is Luminosity and What does it Tell Us? Emission Spectra Identify Elements And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? Each element has its own unique absorption spectra, just like it has a unique emission spectra. Continuous spectra are produced by electrons being shared between. The emission spectra of various atoms. The set of individual colors emitted by an element. Emission Spectra Identify Elements.
From scienceready.com.au
Continuous, Absorption and Emission Spectrum Science Ready Emission Spectra Identify Elements This means they can be used to identify. The set of individual colors emitted by an element is called its spectrum. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Continuous spectra are. Emission Spectra Identify Elements.
From www.nagwa.com
Question Video Identifying a Spectrum as an Emission or Absorption Emission Spectra Identify Elements This means that line spectra can be used to identify elements. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. The emission spectrum (or line spectrum) of a chemical. Emission Spectra Identify Elements.
From brokeasshome.com
periodic table emission spectra Emission Spectra Identify Elements The set of individual colors emitted by an element is called its spectrum. The emission spectra of various atoms. This means they can be used to identify. Since the spectrum of each element is unique, spectra can be used like fingerprints to identify. Each element has its own unique absorption spectra, just like it has a unique emission spectra. The. Emission Spectra Identify Elements.
From umop.net
Visible Spectra of the Elements Emission Spectra Identify Elements This means they can be used to identify. This means that line spectra can be used to identify elements. Continuous spectra are produced by electrons being shared between. The set of individual colors emitted by an element is called its spectrum. Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary. Emission Spectra Identify Elements.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Emission Spectra Identify Elements The set of individual colors emitted by an element is called its spectrum. And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? Any given element therefore has both a characteristic emission spectrum and a characteristic absorption spectrum, which are essentially complementary images. The emission spectrum (or line spectrum) of. Emission Spectra Identify Elements.
From mungfali.com
Atomic Emission Spectrum Of Elements Emission Spectra Identify Elements Each element has its own unique absorption spectra, just like it has a unique emission spectra. The set of individual colors emitted by an element is called its spectrum. The emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat. This means that line spectra can be. Emission Spectra Identify Elements.