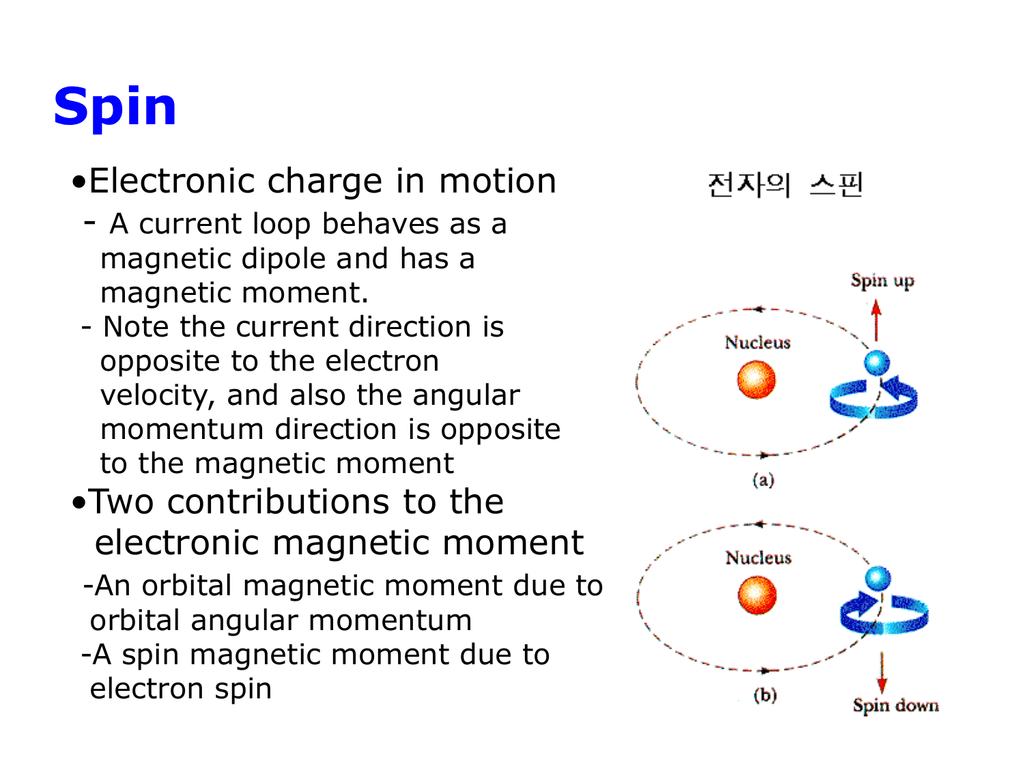
Magnetism With Electrons . Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. The simplest “atomic” system is a single electron. The atoms in ferromagnetic materials act. It can be an electric current in a conductor or charged particles moving. The unpaired electrons are attracted by a magnetic. As the electrons move relative to protons (ions). Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. All magnetism is created by electric current. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. It requires electrons and protons to produce a magnetic field. A moving electron alone actually does not produce a magnetic field. The spin of an electron is $1/2$, so there are two possible states: We call this motion the electron spin.
from magworld.physics.auth.gr
As the electrons move relative to protons (ions). All magnetism is created by electric current. A moving electron alone actually does not produce a magnetic field. Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. The unpaired electrons are attracted by a magnetic. The simplest “atomic” system is a single electron. It can be an electric current in a conductor or charged particles moving. Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. The spin of an electron is $1/2$, so there are two possible states:
World World
Magnetism With Electrons Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. It requires electrons and protons to produce a magnetic field. We call this motion the electron spin. Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. The spin of an electron is $1/2$, so there are two possible states: It can be an electric current in a conductor or charged particles moving. The simplest “atomic” system is a single electron. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. A moving electron alone actually does not produce a magnetic field. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. As the electrons move relative to protons (ions). All magnetism is created by electric current. The atoms in ferromagnetic materials act. Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. The unpaired electrons are attracted by a magnetic.
From www.sciencenews.org
Electrons’ confirms particle physics’ most precise prediction Magnetism With Electrons All magnetism is created by electric current. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. The atoms in ferromagnetic materials act. It can be an electric current in a conductor or charged particles moving. The unpaired electrons are attracted by a magnetic. A moving electron. Magnetism With Electrons.
From www.resonancescience.org
The Electron Moment and its Significance for Standard Model Magnetism With Electrons We call this motion the electron spin. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. It can be an electric current in a conductor or charged particles moving. It requires electrons and protons to produce a magnetic field. Paramagnetism refers to the. Magnetism With Electrons.
From www.wonkeedonkeetools.co.uk
How does a work? Magnetism With Electrons A moving electron alone actually does not produce a magnetic field. The spin of an electron is $1/2$, so there are two possible states: It requires electrons and protons to produce a magnetic field. The atoms in ferromagnetic materials act. As the electrons move relative to protons (ions). The unpaired electrons are attracted by a magnetic. We call this motion. Magnetism With Electrons.
From www.physicsforums.com
Fundamental fields generated by moving electrons Magnetism With Electrons The spin of an electron is $1/2$, so there are two possible states: All magnetism is created by electric current. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. It can be an electric current in a conductor or charged particles moving. As the electrons move relative to protons (ions). The atoms in ferromagnetic materials. Magnetism With Electrons.
From sciencenotes.org
vs vs Magnetism With Electrons Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. We call this motion the electron spin. It requires electrons and protons to produce a magnetic field. As the electrons move relative to protons (ions). A moving electron alone actually does not produce a magnetic field. The atoms in ferromagnetic materials act. The simplest “atomic”. Magnetism With Electrons.
From www.resonancescience.org
The Electron Moment and its Significance for Standard Model Magnetism With Electrons We call this motion the electron spin. It can be an electric current in a conductor or charged particles moving. A moving electron alone actually does not produce a magnetic field. The simplest “atomic” system is a single electron. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some. Magnetism With Electrons.
From www.snexplores.org
Scientists Say Magnetism With Electrons Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. As the electrons move relative to protons (ions). It requires electrons and protons to produce a magnetic field. The simplest “atomic” system is a single electron. The unpaired electrons are attracted by a magnetic. A moving electron alone actually does not produce a magnetic field. We. Magnetism With Electrons.
From app.jove.com
Moment of an Electron Physics JoVe Magnetism With Electrons It can be an electric current in a conductor or charged particles moving. As the electrons move relative to protons (ions). It requires electrons and protons to produce a magnetic field. The simplest “atomic” system is a single electron. A moving electron alone actually does not produce a magnetic field. The unpaired electrons are attracted by a magnetic. Paramagnetism refers. Magnetism With Electrons.
From quizizz.com
Science Quiz Quizizz Magnetism With Electrons It can be an electric current in a conductor or charged particles moving. We call this motion the electron spin. Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. The simplest. Magnetism With Electrons.
From physicsworld.com
Electrons' interactions isolated at long last Magnetism With Electrons All magnetism is created by electric current. A moving electron alone actually does not produce a magnetic field. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. The atoms in ferromagnetic materials act. Ferromagnetic materials, such as iron, are those. Magnetism With Electrons.
From www.researchgate.net
Types of a) b) c Magnetism With Electrons The simplest “atomic” system is a single electron. All magnetism is created by electric current. Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. The spin of an electron is $1/2$, so there are two possible states: As the electrons move relative to protons (ions). Paramagnetism refers to the magnetic state of an atom with one or. Magnetism With Electrons.
From 9to5science.com
[Solved] What is the moment, and what does it 9to5Science Magnetism With Electrons Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. All magnetism is created by electric current. The spin of an electron is $1/2$, so there are two possible states: Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric. Magnetism With Electrons.
From ecuip.lib.uchicago.edu
Force Multiwavelength Astronomy Magnetism With Electrons Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. It requires electrons and protons to produce a magnetic field. The unpaired electrons are attracted by a magnetic. A moving electron alone actually does not produce a magnetic field. It can be an electric current in a conductor or charged particles moving. Since an electron may exhibit a. Magnetism With Electrons.
From energywavetheory.com
Force EWT Magnetism With Electrons As the electrons move relative to protons (ions). It requires electrons and protons to produce a magnetic field. The atoms in ferromagnetic materials act. A moving electron alone actually does not produce a magnetic field. The unpaired electrons are attracted by a magnetic. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum,. Magnetism With Electrons.
From kwantumrevolutie.wtnschp.be
7.11 De tabel van Mendeljev Kwantumrevolutie Magnetism With Electrons A moving electron alone actually does not produce a magnetic field. We call this motion the electron spin. Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. It can be an electric current in a conductor or charged particles moving. The spin of an electron is $1/2$, so there are two possible states: The. Magnetism With Electrons.
From www.youtube.com
Field of a Moving Charge, Proton, Right Hand Rule Physics Magnetism With Electrons The simplest “atomic” system is a single electron. A moving electron alone actually does not produce a magnetic field. It requires electrons and protons to produce a magnetic field. Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. The spin of an electron is $1/2$, so there are two possible states: It can be an electric current. Magnetism With Electrons.
From educatorpages.com
Magnetism With Electrons Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. The simplest “atomic” system is a single electron. All magnetism is created by electric current. It can be an electric current in a. Magnetism With Electrons.
From funsizephysics.com
A Molecular Switch FunsizePhysics Magnetism With Electrons The unpaired electrons are attracted by a magnetic. All magnetism is created by electric current. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. A moving electron alone actually does not produce a magnetic field. Magnetism, phenomenon associated with magnetic fields, which arise from the motion. Magnetism With Electrons.
From www.youtube.com
ORBITAL MOMENT OF AN ELECTRON REVOLVING IN AN ORBIT OF AN ATOM Magnetism With Electrons The unpaired electrons are attracted by a magnetic. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. The spin of an electron is $1/2$, so there are two possible states: It requires electrons and protons to produce a magnetic field. A moving electron alone actually does not produce a magnetic field. Paramagnetism refers to the. Magnetism With Electrons.
From www.slideserve.com
PPT Inside a PowerPoint Presentation, free download ID5520463 Magnetism With Electrons The simplest “atomic” system is a single electron. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. It requires electrons and protons to produce a magnetic field. The unpaired electrons are attracted by a magnetic. It can be an electric. Magnetism With Electrons.
From www.sciencelearn.org.nz
field — Science Learning Hub Magnetism With Electrons Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. All magnetism is created by electric current. Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. The unpaired electrons are attracted by a magnetic. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. It can be an. Magnetism With Electrons.
From physics-12th.blogspot.com
Physics 12 Field and Force Magnetism With Electrons The spin of an electron is $1/2$, so there are two possible states: The atoms in ferromagnetic materials act. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. It can be an electric current in a conductor or charged particles moving. A moving electron alone actually. Magnetism With Electrons.
From musicbykatie.com
Do Electrically Charged Particles Interact With Fields? The 15 Magnetism With Electrons The simplest “atomic” system is a single electron. All magnetism is created by electric current. Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. It can be an electric current in a conductor or charged particles moving. The spin of an electron is $1/2$, so there are two possible states: Since an electron may. Magnetism With Electrons.
From www.expii.com
Electron Spin — Definition & Overview Expii Magnetism With Electrons The atoms in ferromagnetic materials act. All magnetism is created by electric current. We call this motion the electron spin. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. A moving electron alone actually does not produce a magnetic field. Paramagnetism refers to the magnetic state. Magnetism With Electrons.
From www.slideserve.com
PPT New Measurement of the Electron Moment and the Fine Magnetism With Electrons It can be an electric current in a conductor or charged particles moving. It requires electrons and protons to produce a magnetic field. A moving electron alone actually does not produce a magnetic field. We call this motion the electron spin. All magnetism is created by electric current. The unpaired electrons are attracted by a magnetic. The simplest “atomic” system. Magnetism With Electrons.
From materials-mag-algerien970.blogspot.com
and Properties of Materials Magnetism With Electrons The simplest “atomic” system is a single electron. A moving electron alone actually does not produce a magnetic field. The unpaired electrons are attracted by a magnetic. Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. All magnetism is created by electric current. Paramagnetism refers to. Magnetism With Electrons.
From www.slideserve.com
PPT PowerPoint Presentation ID2795990 Magnetism With Electrons The atoms in ferromagnetic materials act. We call this motion the electron spin. The simplest “atomic” system is a single electron. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. A moving electron alone actually does not produce a magnetic field. The unpaired electrons are attracted by a magnetic. As the electrons move relative to. Magnetism With Electrons.
From scitechdaily.com
Physicists Measure Interactions between Single Electrons Magnetism With Electrons As the electrons move relative to protons (ions). Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. A moving electron alone actually does not produce a magnetic field. It can be an electric current in a conductor or charged particles moving. The simplest “atomic” system is. Magnetism With Electrons.
From www.researchgate.net
Electrons and Protons gyrating around positive Low Line of Magnetism With Electrons The atoms in ferromagnetic materials act. We call this motion the electron spin. It can be an electric current in a conductor or charged particles moving. The spin of an electron is $1/2$, so there are two possible states: As the electrons move relative to protons (ions). The unpaired electrons are attracted by a magnetic. Ferromagnetic materials, such as iron,. Magnetism With Electrons.
From sciencenotes.org
vs vs Magnetism With Electrons Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. The atoms in ferromagnetic materials act. As the electrons move relative to protons (ions). Ferromagnetic materials, such as iron, are those that exhibit strong magnetic effects. The simplest “atomic” system is a single electron. The unpaired electrons. Magnetism With Electrons.
From www.scienceabc.com
Is Aluminum What Causes Magnetism With Electrons All magnetism is created by electric current. A moving electron alone actually does not produce a magnetic field. It requires electrons and protons to produce a magnetic field. We call this motion the electron spin. As the electrons move relative to protons (ions). Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. The simplest. Magnetism With Electrons.
From www.slideserve.com
PPT The Cause of PowerPoint Presentation, free download Magnetism With Electrons The simplest “atomic” system is a single electron. A moving electron alone actually does not produce a magnetic field. The spin of an electron is $1/2$, so there are two possible states: It requires electrons and protons to produce a magnetic field. The atoms in ferromagnetic materials act. Paramagnetism refers to the magnetic state of an atom with one or. Magnetism With Electrons.
From askfilo.com
The diagram shows a beam of electrons entering a field. What is.. Magnetism With Electrons Since an electron may exhibit a magnetic moment even when it does not possess orbital angular momentum, it must possess some internal motion. The unpaired electrons are attracted by a magnetic. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. We call this motion the electron spin. The atoms in ferromagnetic materials act. A moving. Magnetism With Electrons.
From magworld.physics.auth.gr
World World Magnetism With Electrons As the electrons move relative to protons (ions). A moving electron alone actually does not produce a magnetic field. The simplest “atomic” system is a single electron. Magnetism, phenomenon associated with magnetic fields, which arise from the motion of electric charges. It can be an electric current in a conductor or charged particles moving. Ferromagnetic materials, such as iron, are. Magnetism With Electrons.
From learningcampusdoggy.z21.web.core.windows.net
Describe Electricity And Magnetism With Electrons The spin of an electron is $1/2$, so there are two possible states: It requires electrons and protons to produce a magnetic field. The simplest “atomic” system is a single electron. The atoms in ferromagnetic materials act. As the electrons move relative to protons (ions). The unpaired electrons are attracted by a magnetic. Ferromagnetic materials, such as iron, are those. Magnetism With Electrons.