Sterilization Validation For Medical Devices . Confirm that the sterilization process was validated by reviewing the validation study. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Review the specific procedure (s) for the sterilization process. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical.
from www.cirs-group.com
Sterilization of health care products — radiation — part 4: Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Confirm that the sterilization process was validated by reviewing the validation study. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Review the specific procedure (s) for the sterilization process. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply.
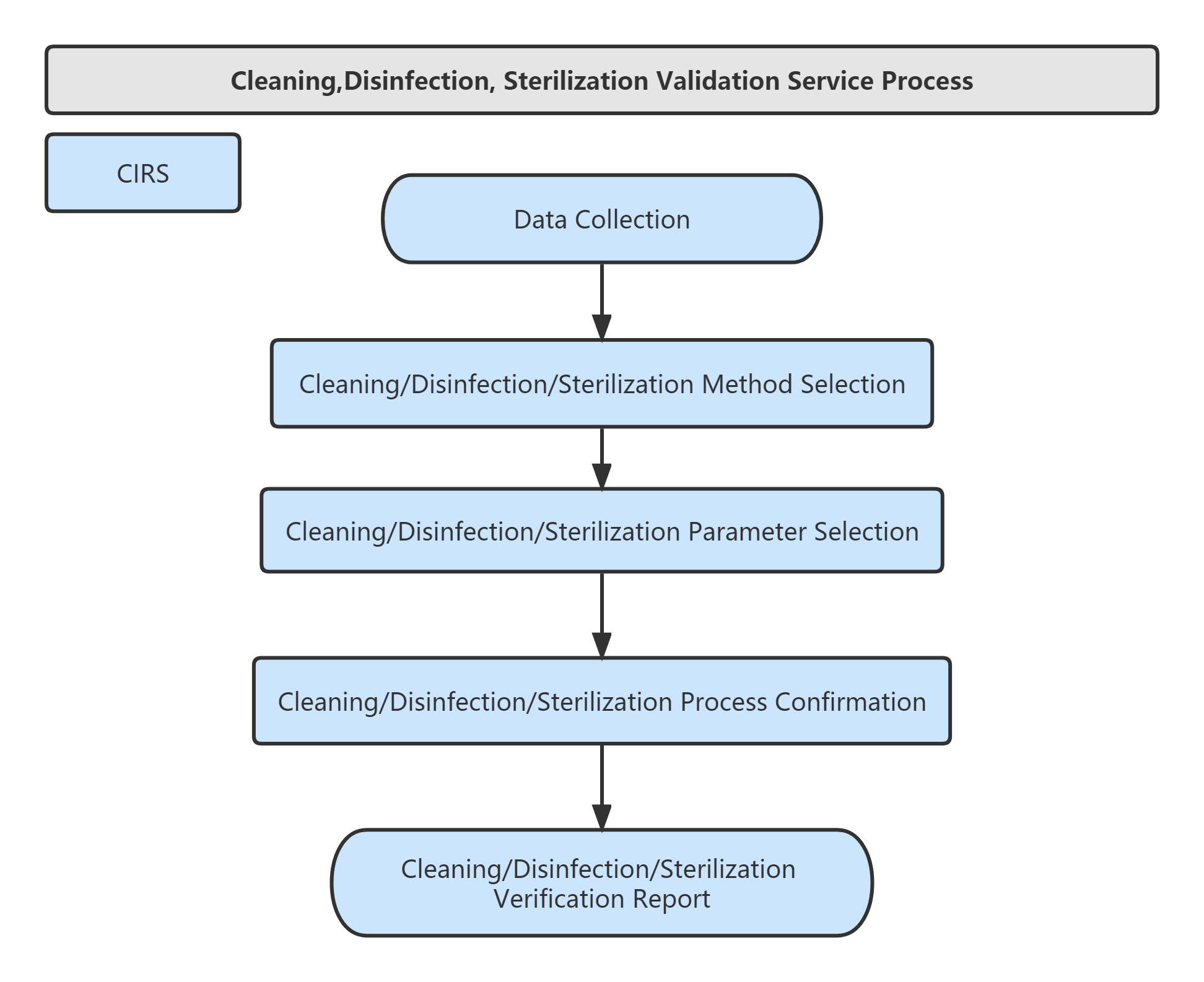
Validation of Cleaning, Disinfection, Sterilization Validation of
Sterilization Validation For Medical Devices Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Confirm that the sterilization process was validated by reviewing the validation study. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Review the specific procedure (s) for the sterilization process. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Sterilization of health care products — radiation — part 4:
From mavenprofserv.com
ETO Sterilization Effective Methods & Best Practices Sterilization Validation For Medical Devices A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide. Sterilization Validation For Medical Devices.
From joiratjtk.blob.core.windows.net
Applications Of Sterilization at Glen Paulus blog Sterilization Validation For Medical Devices Review the specific procedure (s) for the sterilization process. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. Sterilization of health care products — radiation — part 4: Confirm that the sterilization process. Sterilization Validation For Medical Devices.
From www.sanichem.my
Sterilization Validation for Medical Devices Navigating ISO 11135 and Sterilization Validation For Medical Devices Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Sterilization of health care products — radiation — part 4: Review the specific procedure (s) for the sterilization process. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. A sterilization. Sterilization Validation For Medical Devices.
From www.steris.in
STERIS India's First Outsourcing ETO Sterilization Service Sterilization Validation For Medical Devices Sterilization of health care products — radiation — part 4: Confirm that the sterilization process was validated by reviewing the validation study. Review the specific procedure (s) for the sterilization process. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Our sterilization and microbiology testing services for medical devices provide. Sterilization Validation For Medical Devices.
From info.mesalabs.com
Pharmaceutical Aseptic Manufacturing Sterilization Validation Mesa Labs Sterilization Validation For Medical Devices A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Sterilization of health care products —. Sterilization Validation For Medical Devices.
From pathwaynpi.com
Medical Device Sterilization How To Choose the Right Method Pathway Sterilization Validation For Medical Devices A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. Confirm that the sterilization process was validated by reviewing the validation study. International standards that specify requirements for validation and routine control of sterilization. Sterilization Validation For Medical Devices.
From qbdgroup.com
Sterilization Validation Ensuring Safety and Compliance Sterilization Validation For Medical Devices Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Confirm. Sterilization Validation For Medical Devices.
From old.sermitsiaq.ag
Medical Device Verification And Validation Plan Template Sterilization Validation For Medical Devices International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Sterilization of health care products — radiation — part 4: Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Medical devices are sterilized in a variety of ways including using. Sterilization Validation For Medical Devices.
From lso-inc.com
6 Methods for Medical Device Sterilization How They Work and Which Sterilization Validation For Medical Devices Sterilization of health care products — radiation — part 4: Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Review the specific procedure (s) for the sterilization process. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Confirm that the. Sterilization Validation For Medical Devices.
From www.slideserve.com
PPT Validating Sterilization of Medical Devices PowerPoint Sterilization Validation For Medical Devices International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Medical devices are sterilized in a variety of ways including using. Sterilization Validation For Medical Devices.
From www.youtube.com
Guidance for Cleaning, Disinfection and Sterilization of Reusable Sterilization Validation For Medical Devices A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat,. Sterilization Validation For Medical Devices.
From www.researchgate.net
(PDF) Process Validation and Revalidation in Medical Device Production Sterilization Validation For Medical Devices Sterilization of health care products — radiation — part 4: Review the specific procedure (s) for the sterilization process. Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. International standards that specify requirements for validation. Sterilization Validation For Medical Devices.
From pacificbiolabs.com
Sterilization Validations Pacific BioLabs Sterilization Validation For Medical Devices Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. International standards that specify requirements for validation and routine control. Sterilization Validation For Medical Devices.
From www.tapecon.com
Clean and Cleared Most Common Sterilization Methods for Printed Sterilization Validation For Medical Devices Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Review the specific procedure (s) for the sterilization process. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. International standards that specify requirements for validation. Sterilization Validation For Medical Devices.
From highpowervtls.com
Medical Device Cleaning vs. Sterilization Validation Sterilization Validation For Medical Devices Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Confirm that. Sterilization Validation For Medical Devices.
From www.i3cglobal.com
Sterilization Validation For Medical Devices I3CGLOBAL Sterilization Validation For Medical Devices International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. Review the specific procedure (s) for the sterilization process. Confirm that the sterilization process was validated by reviewing the. Sterilization Validation For Medical Devices.
From www.cannondigi.com
Iso 11137 Gamma E Beam Sterilization For Medical Devices The Best Sterilization Validation For Medical Devices A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Confirm that the sterilization process was validated by reviewing the validation study. Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Our sterilization and microbiology. Sterilization Validation For Medical Devices.
From www.slideserve.com
PPT Validating Sterilization of Medical Devices PowerPoint Sterilization Validation For Medical Devices Review the specific procedure (s) for the sterilization process. Sterilization of health care products — radiation — part 4: Confirm that the sterilization process was validated by reviewing the validation study. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. A sterilization validation test confirms the appropriate sterility. Sterilization Validation For Medical Devices.
From www.sterilizationconsulting.com
sterilization validation Sterilization Validation For Medical Devices Review the specific procedure (s) for the sterilization process. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Our sterilization and microbiology testing services for medical devices provide manufacturers with. Sterilization Validation For Medical Devices.
From medicaldeviceacademy.com
510k Submission, Section 14Sterilization Validation and Shelflife Sterilization Validation For Medical Devices International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Sterilization of health care products — radiation — part 4: Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. A sterilization validation test confirms the appropriate sterility assurance level (sal). Sterilization Validation For Medical Devices.
From ethidelabs.com
Ethide Laboratories sterilization validation methods Archives Sterilization Validation For Medical Devices A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Review the specific procedure (s) for the sterilization process. Sterilization of health care products — radiation — part 4: Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. International standards that specify requirements. Sterilization Validation For Medical Devices.
From highpowervtls.com
What Is a Steam Sterilization Efficacy Validation for Medical Devices? Sterilization Validation For Medical Devices International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Review the specific procedure (s) for the sterilization process. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Medical devices are sterilized in a variety of ways including using moist. Sterilization Validation For Medical Devices.
From gemarmed.com
Medical Device Sterilization Validation Services Gemarmed Sterilization Validation For Medical Devices International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Review the specific procedure (s) for the sterilization process. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. A sterilization validation test confirms the appropriate sterility assurance level. Sterilization Validation For Medical Devices.
From www.cirs-group.com
Validation of Cleaning, Disinfection, Sterilization Validation of Sterilization Validation For Medical Devices Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Our sterilization and microbiology testing services. Sterilization Validation For Medical Devices.
From www.aplyon.com
Gamma Irradiation Sterilization Validation Procedure Sterilization Validation For Medical Devices Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. Review the specific procedure (s) for the sterilization process. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Confirm that the sterilization process was validated by reviewing the validation study. Our sterilization and. Sterilization Validation For Medical Devices.
From medicaldeviceacademy.com
Generic Sterilization Process Flow Diagram Medical Device Academy Sterilization Validation For Medical Devices Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Confirm that the sterilization process was validated by reviewing the validation study. Sterilization of health care products — radiation — part 4: Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or.. Sterilization Validation For Medical Devices.
From www.qualitymeddev.com
Gamma Sterilization Process for Medical Devices QualityMedDev Sterilization Validation For Medical Devices International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Review the specific procedure (s) for the sterilization process. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat,. Sterilization Validation For Medical Devices.
From www.slideserve.com
PPT Validating Sterilization of Medical Devices PowerPoint Sterilization Validation For Medical Devices Review the specific procedure (s) for the sterilization process. Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the. Sterilization Validation For Medical Devices.
From www.slideserve.com
PPT Validating Sterilization of Medical Devices PowerPoint Sterilization Validation For Medical Devices Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Confirm that the sterilization process was validated by reviewing the validation study. Medical devices are sterilized in a variety of ways including using moist heat (steam),. Sterilization Validation For Medical Devices.
From www.slideserve.com
PPT Validating Sterilization of Medical Devices PowerPoint Sterilization Validation For Medical Devices Review the specific procedure (s) for the sterilization process. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. Sterilization of health care products — radiation — part 4: Confirm that. Sterilization Validation For Medical Devices.
From aplyonqms.com
Ethylene Oxide Sterilization Validation Procedure A. P. LYON Sterilization Validation For Medical Devices Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. Review the specific procedure (s) for the sterilization process. Confirm that the sterilization process was validated by reviewing the validation study. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Medical devices are sterilized in. Sterilization Validation For Medical Devices.
From novasterilis.com
Sterilization Validation Summary & Checklist NovaSterilis Sterilization Validation For Medical Devices Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Medical devices are sterilized in a variety of ways including using. Sterilization Validation For Medical Devices.
From www.rsd-engineering.com
Protocols and validation EtO sterilization equipment RSD sterilization Sterilization Validation For Medical Devices Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Medical devices are sterilized in a variety of ways including using moist heat (steam), dry heat, radiation, ethylene oxide gas, vaporized. International standards that specify requirements. Sterilization Validation For Medical Devices.
From www.slideserve.com
PPT Why LowTemperature? PowerPoint Presentation, free download ID Sterilization Validation For Medical Devices Sterilization of health care products — radiation — part 4: Iso 11135:2014 specifies requirements for the development, validation and routine control of an ethylene oxide sterilization process for medical. International standards that specify requirements for validation and routine control of sterilization processes require, when it is necessary to supply. Medical devices are sterilized in a variety of ways including using. Sterilization Validation For Medical Devices.
From www.medicaldesignbriefs.com
Reusable Medical Devices BI Selection, Overkill Validation Approaches Sterilization Validation For Medical Devices Our sterilization and microbiology testing services for medical devices provide manufacturers with product insight and validation to eliminate or. A sterilization validation test confirms the appropriate sterility assurance level (sal) of the medical device. Confirm that the sterilization process was validated by reviewing the validation study. Medical devices are sterilized in a variety of ways including using moist heat (steam),. Sterilization Validation For Medical Devices.