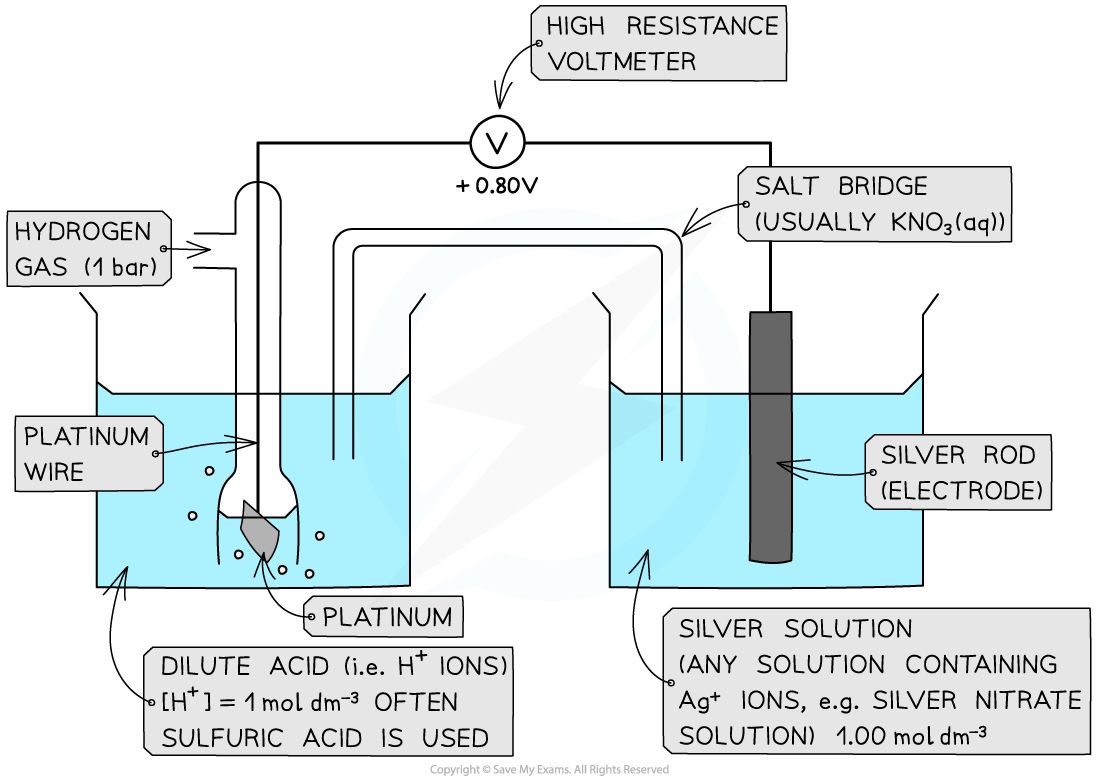
Standard Cell Potential Is Measured . The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential can be determined by two methods: The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant strengths. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the.
from www.hanlin.com
The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell.
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode
Standard Cell Potential Is Measured The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential can be determined by two methods: The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. Interpret electrode potentials in terms of relative oxidant and reductant strengths.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Standard Cell Potential Is Measured The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The standard cell potential can be determined by two methods: Describe and relate the definitions of electrode and cell potentials. The standard cell potential (e°cell) is measured at 298 k and all components in their standard. Standard Cell Potential Is Measured.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Cell Potential Is Measured The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. The standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for. Standard Cell Potential Is Measured.
From www.numerade.com
SOLVED EXPERIMENT 1 Using the table of standard reduction potentials Standard Cell Potential Is Measured The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential of the cell under standard conditions. Standard Cell Potential Is Measured.
From www.tpsearchtool.com
Standard Reduction Potential Charts For Chemistry Images Standard Cell Potential Is Measured 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate. Standard Cell Potential Is Measured.
From mavink.com
How To Calculate Cell Potential Standard Cell Potential Is Measured The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential can be determined by two methods: The standard. Standard Cell Potential Is Measured.
From scienceinfo.com
Cell Potential & Standard Cell Potential Standard Cell Potential Is Measured Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential can be determined by two methods: The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as. Standard Cell Potential Is Measured.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Standard Cell Potential Is Measured Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential can be determined by two methods: The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The potential of the cell under standard conditions. Standard Cell Potential Is Measured.
From www.youtube.com
Standard Cell Potentials YouTube Standard Cell Potential Is Measured The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant strengths. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The cell potential, \(e_{cell}\), is the measure of. Standard Cell Potential Is Measured.
From www.hanlin.com
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Standard Cell Potential Is Measured The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells. Standard Cell Potential Is Measured.
From dxoasuand.blob.core.windows.net
Calculate The Standard Cell Potentials Given The Following Data at Standard Cell Potential Is Measured Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. 1 atm for gases, 1 m for. Standard Cell Potential Is Measured.
From www.youtube.com
Calculating Standard Cell Potential YouTube Standard Cell Potential Is Measured Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids. Standard Cell Potential Is Measured.
From f15.beauty
Standard Reduction Potential Table Standard Cell Potential Is Measured The standard cell potential can be determined by two methods: Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. The standard cell potential (e°cell) is measured at 298 k and all components in their. Standard Cell Potential Is Measured.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Is Measured 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. The standard cell potential (e°cell) is measured at 298 k and all components in. Standard Cell Potential Is Measured.
From www.numerade.com
SOLVED Part V Calculate Cell Potential Under Nonstandard Condition Standard Cell Potential Is Measured The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions. Standard Cell Potential Is Measured.
From wisc.pb.unizin.org
Day 39 Voltaic Cells, HalfCell Potentials Chemistry 109 Fall 2021 Standard Cell Potential Is Measured The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under. Standard Cell Potential Is Measured.
From www.nagwa.com
Question Video Calculating the Standard Cell Potential for a Copper Standard Cell Potential Is Measured The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf measured when a metal / metal ion. Standard Cell Potential Is Measured.
From www.slideserve.com
PPT REDOX REACTIONS PowerPoint Presentation, free download ID4348855 Standard Cell Potential Is Measured The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard. Standard Cell Potential Is Measured.
From 2012books.lardbucket.org
Standard Potentials Standard Cell Potential Is Measured Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the.. Standard Cell Potential Is Measured.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Cell Potential Is Measured The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The cell potential, \(e_{cell}\), is the measure of the potential difference. Standard Cell Potential Is Measured.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Is Measured The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. Describe. Standard Cell Potential Is Measured.
From dxorswgth.blob.core.windows.net
Standard Cell Potentials at Shantell Sirmans blog Standard Cell Potential Is Measured The standard cell potential can be determined by two methods: The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under. Standard Cell Potential Is Measured.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Standard Cell Potential Is Measured The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The standard cell potential can be determined by two methods: The potential of the cell under standard. Standard Cell Potential Is Measured.
From chemistryexplainedpsychokillerblogger.blogspot.com
Chemistry Explained Electrode Potential (Introduction) Standard Cell Potential Is Measured Interpret electrode potentials in terms of relative oxidant and reductant strengths. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids. Standard Cell Potential Is Measured.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Is Measured The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \(e_{cell}\), is the measure of. Standard Cell Potential Is Measured.
From www.flinnsci.ca
Measuring Cell Potentials Standard Reduction Potentials—ChemTopic™ Lab Standard Cell Potential Is Measured Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard. Standard Cell Potential Is Measured.
From chem.libretexts.org
6.5 Standard Reduction Potentials Chemistry LibreTexts Standard Cell Potential Is Measured Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The standard cell potential (e°cell) is measured at 298 k and. Standard Cell Potential Is Measured.
From mungfali.com
Electrode Potential Series Standard Cell Potential Is Measured The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states:. Standard Cell Potential Is Measured.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Is Measured The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The cell potential, \(e_{cell}\),. Standard Cell Potential Is Measured.
From www.researchgate.net
a Typical current vs potential curve of an electrochemical cell. Small Standard Cell Potential Is Measured The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. 1 atm for gases,. Standard Cell Potential Is Measured.
From www.slideserve.com
PPT Chapter 22 Electrochemistry PowerPoint Presentation, free Standard Cell Potential Is Measured The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential. Standard Cell Potential Is Measured.
From cemubadx.blob.core.windows.net
Standard Cell Potential And Standard Electrode Potential at Nina Standard Cell Potential Is Measured The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called the. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: 1 atm for gases, 1 m for solutions, and the pure. Standard Cell Potential Is Measured.
From cemubadx.blob.core.windows.net
Standard Cell Potential And Standard Electrode Potential at Nina Standard Cell Potential Is Measured The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant strengths. The standard cell potential can be determined by two methods: The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other. Standard Cell Potential Is Measured.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Is Measured The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The standard cell potential can be determined by two methods: Interpret electrode potentials in terms of relative oxidant and reductant strengths. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and. Standard Cell Potential Is Measured.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Cell Potential Is Measured The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant strengths. Describe and relate the definitions of electrode and cell potentials. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \(e_{cell}\), is the measure of. Standard Cell Potential Is Measured.
From www.slideserve.com
PPT Standard Reference Electrode Standard Hydrogen Electrode (SHE Standard Cell Potential Is Measured The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. The emf measured when a metal / metal ion electrode is coupled to a. Standard Cell Potential Is Measured.