What Is Actual Value In Chemistry . If you’re in a lab for a chemistry course, chances are. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. Subtract the accepted value from the experimental value. Measurements may be accurate, meaning that the measured value is the same as the true value; The problem is you can’t always know what the actual value is. Accuracy is how close your value is to the true value. Percent error is most frequently used in chemistry and the. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. Steps to calculate the percent error. The maximum possible mass of a product that a chemical reaction can make. Take the absolute value of step 1. In general, error is the difference between an accepted or theoretical value and an. Here are the most common ways to calculate experimental error: It is calculated using molar ratios.
from www.youtube.com
Take the absolute value of step 1. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. It is calculated using molar ratios. Subtract the accepted value from the experimental value. Here are the most common ways to calculate experimental error: Percent error is most frequently used in chemistry and the. If you’re in a lab for a chemistry course, chances are. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. The problem is you can’t always know what the actual value is. Steps to calculate the percent error.
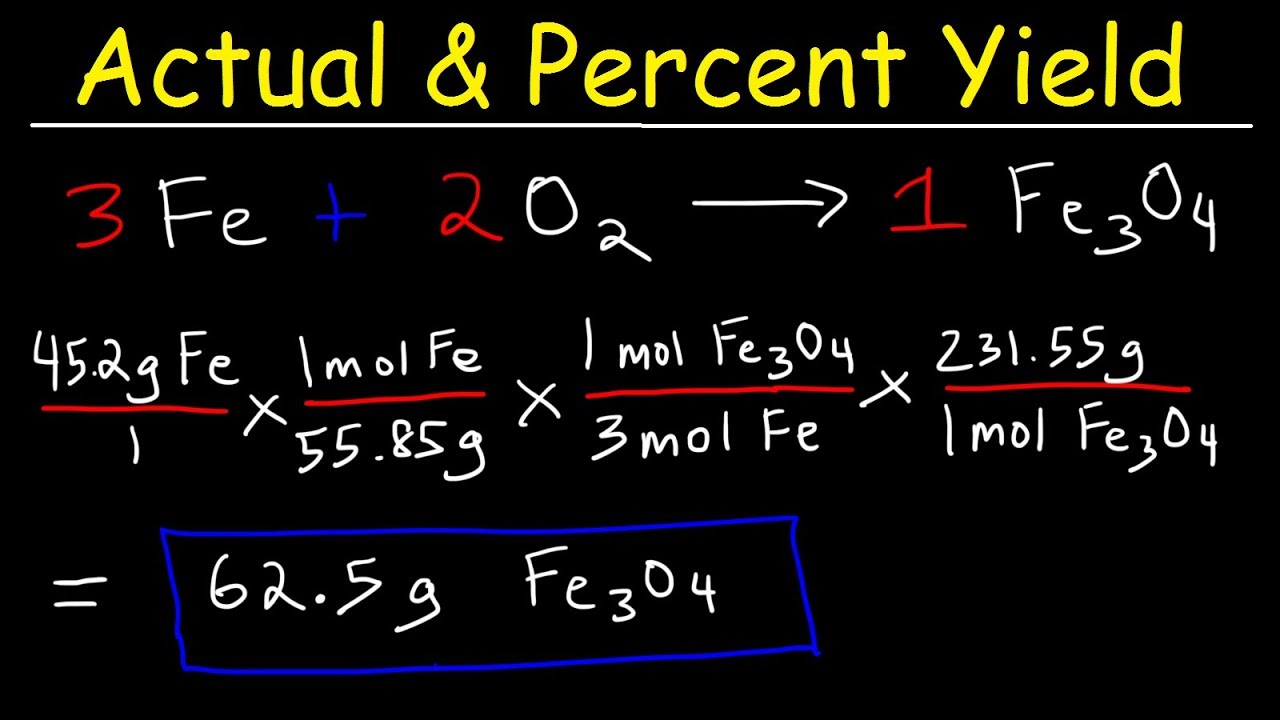
How To Calculate The Percent Yield and Theoretical Yield YouTube
What Is Actual Value In Chemistry Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. They may be precise, meaning that multiple measurements give nearly identical values (i.e.,. The problem is you can’t always know what the actual value is. Subtract the accepted value from the experimental value. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. Here are the most common ways to calculate experimental error: The maximum possible mass of a product that a chemical reaction can make. Steps to calculate the percent error. Take the absolute value of step 1. Measurements may be accurate, meaning that the measured value is the same as the true value; Percent error is most frequently used in chemistry and the. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. It is calculated using molar ratios. Accuracy is how close your value is to the true value. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. If you’re in a lab for a chemistry course, chances are.
From ar.inspiredpencil.com
Percent Error Formula Chemistry What Is Actual Value In Chemistry It is calculated using molar ratios. They may be precise, meaning that multiple measurements give nearly identical values (i.e.,. Here are the most common ways to calculate experimental error: Percent error is most frequently used in chemistry and the. Take the absolute value of step 1. In general, error is the difference between an accepted or theoretical value and an.. What Is Actual Value In Chemistry.
From adysonancepruitt.blogspot.com
How to Calculate Yield AdysonancePruitt What Is Actual Value In Chemistry It is calculated using molar ratios. The problem is you can’t always know what the actual value is. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. The maximum possible mass of a product that a chemical reaction can make. Accuracy is how close your value is to. What Is Actual Value In Chemistry.
From www.slideshare.net
Calculating percent error What Is Actual Value In Chemistry Measurements may be accurate, meaning that the measured value is the same as the true value; In general, error is the difference between an accepted or theoretical value and an. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. Here are. What Is Actual Value In Chemistry.
From www.tessshebaylo.com
What Is A Percent Equation Definition Tessshebaylo What Is Actual Value In Chemistry If you’re in a lab for a chemistry course, chances are. The maximum possible mass of a product that a chemical reaction can make. It is calculated using molar ratios. Here are the most common ways to calculate experimental error: The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given. What Is Actual Value In Chemistry.
From www.youtube.com
How to Calculate Theoretical Yields YouTube What Is Actual Value In Chemistry It is calculated using molar ratios. The maximum possible mass of a product that a chemical reaction can make. In general, error is the difference between an accepted or theoretical value and an. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. Take the absolute value of. What Is Actual Value In Chemistry.
From www.youtube.com
How To Calculate The Percent Yield and Theoretical Yield YouTube What Is Actual Value In Chemistry They may be precise, meaning that multiple measurements give nearly identical values (i.e.,. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. Measurements may be accurate, meaning that the measured value is the same as the true value; Subtract the accepted value from the experimental value. Here. What Is Actual Value In Chemistry.
From learningzonewalsh.z21.web.core.windows.net
In Chemistry What Is A Hydrate What Is Actual Value In Chemistry The problem is you can’t always know what the actual value is. Take the absolute value of step 1. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. Subtract the accepted value from the experimental value. The maximum possible mass of a product that a chemical reaction can. What Is Actual Value In Chemistry.
From www.youtube.com
What is Actual Value vs. Replacement Cost? YouTube What Is Actual Value In Chemistry To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. The maximum possible mass of a product that a chemical reaction can make. The problem is you can’t always know what the actual value is. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. Steps to calculate the percent error.. What Is Actual Value In Chemistry.
From www.automationninjas.com
Perceived Value vs Actual Value Automation Ninjas What Is Actual Value In Chemistry It is calculated using molar ratios. Take the absolute value of step 1. Subtract the accepted value from the experimental value. The maximum possible mass of a product that a chemical reaction can make. If you’re in a lab for a chemistry course, chances are. Steps to calculate the percent error. Percent error is most frequently used in chemistry and. What Is Actual Value In Chemistry.
From www.youtube.com
Calculate the Theoretical Yield to determine the yield in a chemical What Is Actual Value In Chemistry Take the absolute value of step 1. Here are the most common ways to calculate experimental error: The maximum possible mass of a product that a chemical reaction can make. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. Measurements may be accurate, meaning that the measured value. What Is Actual Value In Chemistry.
From www.youtube.com
AP Chemistry Hydrate Practice Problem 1 YouTube What Is Actual Value In Chemistry Accuracy is how close your value is to the true value. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. Take the absolute value of step 1. The maximum possible mass of a product that a chemical reaction can make.. What Is Actual Value In Chemistry.
From chemistry.stackexchange.com
thermodynamics Reaction quotient and Gibbs free energy at the start What Is Actual Value In Chemistry Percent error is most frequently used in chemistry and the. In general, error is the difference between an accepted or theoretical value and an. They may be precise, meaning that multiple measurements give nearly identical values (i.e.,. Take the absolute value of step 1. If you’re in a lab for a chemistry course, chances are. To calculate the percent error,. What Is Actual Value In Chemistry.
From www.numerade.com
Is your measurement of absolute zero close to the actual value (273 °C What Is Actual Value In Chemistry Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. The problem is you can’t always know what the actual value is. Percent error is most frequently used in chemistry and the. It is calculated using molar ratios. The maximum possible mass of a product that a chemical reaction can make. Measurements may be accurate, meaning that the measured value is the same. What Is Actual Value In Chemistry.
From ar.inspiredpencil.com
Percent Error Equation What Is Actual Value In Chemistry Steps to calculate the percent error. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. If you’re in a lab for a chemistry course, chances are. Percent error is most frequently used in chemistry and the. The maximum possible mass of a product that a chemical reaction can. What Is Actual Value In Chemistry.
From howtohacks92.blogspot.com
Calculate Percent Error Chemistry Equation For Percent Change In Mass What Is Actual Value In Chemistry The problem is you can’t always know what the actual value is. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. It is calculated using molar ratios. Take the absolute value of step 1. If you’re in a lab for a chemistry course, chances are. Measurements may. What Is Actual Value In Chemistry.
From www.tutordale.com
How To Find Percentage Error In Physics What Is Actual Value In Chemistry The problem is you can’t always know what the actual value is. In general, error is the difference between an accepted or theoretical value and an. They may be precise, meaning that multiple measurements give nearly identical values (i.e.,. Here are the most common ways to calculate experimental error: The standard deviation, σ σ, describes the spread of a data. What Is Actual Value In Chemistry.
From www.researchgate.net
pH values of chemical compounds Download Scientific Diagram What Is Actual Value In Chemistry Here are the most common ways to calculate experimental error: Measurements may be accurate, meaning that the measured value is the same as the true value; Subtract the accepted value from the experimental value. It is calculated using molar ratios. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given. What Is Actual Value In Chemistry.
From sciencenotes.org
Absolute and Relative Error and How to Calculate Them What Is Actual Value In Chemistry Here are the most common ways to calculate experimental error: Accuracy is how close your value is to the true value. If you’re in a lab for a chemistry course, chances are. Measurements may be accurate, meaning that the measured value is the same as the true value; Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. It is calculated using molar. What Is Actual Value In Chemistry.
From www.youtube.com
How To Calculate Percent Error Practice 2 YouTube What Is Actual Value In Chemistry Steps to calculate the percent error. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. The problem is you can’t always know what the actual value is. The maximum possible mass of a product that a chemical reaction can make. Percent error is most frequently used in. What Is Actual Value In Chemistry.
From lessonmagicbroxtowe.z21.web.core.windows.net
How To Solve For Percent Yield What Is Actual Value In Chemistry In general, error is the difference between an accepted or theoretical value and an. The maximum possible mass of a product that a chemical reaction can make. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. The standard deviation, σ σ, describes the spread of a data. What Is Actual Value In Chemistry.
From www.wikihow.com
How to Calculate Theoretical Yield 12 Steps (with Pictures) What Is Actual Value In Chemistry Here are the most common ways to calculate experimental error: They may be precise, meaning that multiple measurements give nearly identical values (i.e.,. If you’re in a lab for a chemistry course, chances are. Measurements may be accurate, meaning that the measured value is the same as the true value; The problem is you can’t always know what the actual. What Is Actual Value In Chemistry.
From www.slideserve.com
PPT Theoretical, actual, and percentage yields PowerPoint What Is Actual Value In Chemistry Subtract the accepted value from the experimental value. In general, error is the difference between an accepted or theoretical value and an. It is calculated using molar ratios. Steps to calculate the percent error. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. The problem is you can’t always know what the actual value is. Measurements may be accurate, meaning that the. What Is Actual Value In Chemistry.
From ksa.mytutorsource.com
Percentage Error Formula, Definition, How to Calculate It! What Is Actual Value In Chemistry The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. The maximum possible mass of a product that a chemical reaction can make. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. Take the absolute value of step 1. It is calculated using molar ratios. Accuracy is how close your value. What Is Actual Value In Chemistry.
From physicscatalyst.com
Percentage Error Definition, formula, and solved examples What Is Actual Value In Chemistry In general, error is the difference between an accepted or theoretical value and an. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. If you’re in a lab for a chemistry course, chances are. Take the absolute value of step. What Is Actual Value In Chemistry.
From study.com
Accepted Value Definition & Formula Video & Lesson Transcript What Is Actual Value In Chemistry Measurements may be accurate, meaning that the measured value is the same as the true value; Percent error is most frequently used in chemistry and the. The problem is you can’t always know what the actual value is. Accuracy is how close your value is to the true value. They may be precise, meaning that multiple measurements give nearly identical. What Is Actual Value In Chemistry.
From erwinnuza.blogspot.com
Types Of Experimental Errors Reasons for Error in a Chemistry What Is Actual Value In Chemistry Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. Accuracy is how close your value is to the true value. Take the absolute value of step 1. The problem is you can’t always know what the actual value is. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. Steps to. What Is Actual Value In Chemistry.
From cristoiublog15.blogspot.com
Calculate Percent Error Chemistry How to Calculate Percent Error What Is Actual Value In Chemistry Measurements may be accurate, meaning that the measured value is the same as the true value; In general, error is the difference between an accepted or theoretical value and an. Percent error is most frequently used in chemistry and the. If you’re in a lab for a chemistry course, chances are. The maximum possible mass of a product that a. What Is Actual Value In Chemistry.
From wou.edu
CH104 Chapter 6 Quantities in Chemical Reactions Chemistry What Is Actual Value In Chemistry Measurements may be accurate, meaning that the measured value is the same as the true value; Here are the most common ways to calculate experimental error: It is calculated using molar ratios. In general, error is the difference between an accepted or theoretical value and an. Accuracy is how close your value is to the true value. The maximum possible. What Is Actual Value In Chemistry.
From www.thoughtco.com
How to Calculate Percent Error What Is Actual Value In Chemistry Measurements may be accurate, meaning that the measured value is the same as the true value; The maximum possible mass of a product that a chemical reaction can make. Take the absolute value of step 1. It is calculated using molar ratios. Here are the most common ways to calculate experimental error: Percent error is most frequently used in chemistry. What Is Actual Value In Chemistry.
From www.youtube.com
Calculating Reaction Yields YouTube What Is Actual Value In Chemistry To calculate the percent error, you take the difference between a value collected in an experiment and a known or exact value. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. The problem is you can’t always know what the actual value is. Subtract the accepted value from. What Is Actual Value In Chemistry.
From www.youtube.com
Theoretical yield experimental yield yield YouTube What Is Actual Value In Chemistry Percent error is most frequently used in chemistry and the. It is calculated using molar ratios. The maximum possible mass of a product that a chemical reaction can make. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. In general, error. What Is Actual Value In Chemistry.
From www.youtube.com
How To Calculate Theoretical Yield and Percent Yield YouTube What Is Actual Value In Chemistry Measurements may be accurate, meaning that the measured value is the same as the true value; The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. The problem is you can’t always know what the actual value is. Take the absolute value of step 1. Accuracy is how close. What Is Actual Value In Chemistry.
From howtohacks92.blogspot.com
Calculate Percent Error Chemistry Equation For Percent Change In Mass What Is Actual Value In Chemistry They may be precise, meaning that multiple measurements give nearly identical values (i.e.,. Σ = ∑i(xi −x¯¯¯¯)2 n − 1−. Percent error is most frequently used in chemistry and the. Here are the most common ways to calculate experimental error: Measurements may be accurate, meaning that the measured value is the same as the true value; In general, error is. What Is Actual Value In Chemistry.
From www.cuemath.com
How to Calculate Percent Error? Concept and Calculation, Meaning What Is Actual Value In Chemistry The problem is you can’t always know what the actual value is. If you’re in a lab for a chemistry course, chances are. Percent error is most frequently used in chemistry and the. Accuracy is how close your value is to the true value. Subtract the accepted value from the experimental value. They may be precise, meaning that multiple measurements. What Is Actual Value In Chemistry.
From howtohacks92.blogspot.com
Calculate Percent Error Chemistry Equation For Percent Change In Mass What Is Actual Value In Chemistry Percent error is most frequently used in chemistry and the. The standard deviation, σ σ, describes the spread of a data set’s individual values about its mean, and is given as. In general, error is the difference between an accepted or theoretical value and an. Measurements may be accurate, meaning that the measured value is the same as the true. What Is Actual Value In Chemistry.