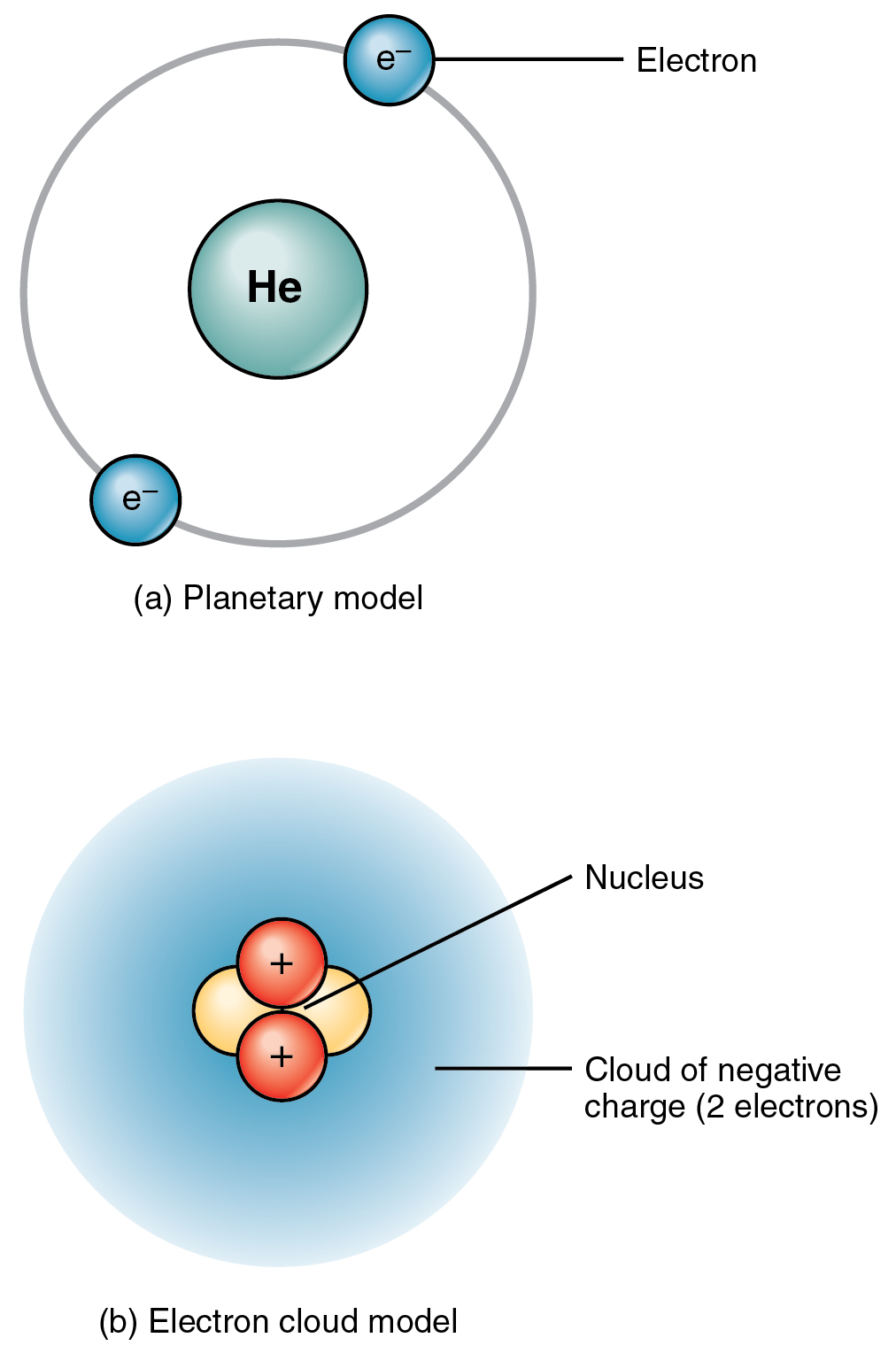
How Do Electrons Form . All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. These free electrons mix with ions to form a plasma. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. They are unimaginably small, so small that. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. Electrons can gain enough energy to leave atomic nuclei behind.
from philschatz.com
Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. These free electrons mix with ions to form a plasma. Electrons can gain enough energy to leave atomic nuclei behind. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. They are unimaginably small, so small that. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom.
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology
How Do Electrons Form Electrons can gain enough energy to leave atomic nuclei behind. These free electrons mix with ions to form a plasma. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. Electrons can gain enough energy to leave atomic nuclei behind. They are unimaginably small, so small that. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom.
From sites.google.com
Structure of the Atom Unit G Review How Do Electrons Form Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. They are unimaginably small, so small that. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. In this. How Do Electrons Form.
From www.mindomo.com
Energy Mind Map How Do Electrons Form They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. Electrons can gain enough energy to leave atomic nuclei behind. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. In this chapter, we describe how electrons are arranged in atoms and how the spatial. How Do Electrons Form.
From www.expii.com
Valence Electrons — Definition & Importance Expii How Do Electrons Form These free electrons mix with ions to form a plasma. Electrons can gain enough energy to leave atomic nuclei behind. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. All atoms have the same number of. How Do Electrons Form.
From www.worksheetsplanet.com
What is an Electron Definition and Characteristics How Do Electrons Form They are unimaginably small, so small that. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Electrons can gain enough energy to leave atomic nuclei behind. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. All atoms have the same number of electrons as protons, so. How Do Electrons Form.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table How Do Electrons Form All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. Electrons are elementary subatomic particles. How Do Electrons Form.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho How Do Electrons Form These free electrons mix with ions to form a plasma. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. In this chapter, we describe how electrons are arranged in atoms and how. How Do Electrons Form.
From courses.lumenlearning.com
Electrons Biology for Majors I How Do Electrons Form They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. They are unimaginably. How Do Electrons Form.
From www.expii.com
Valence Electrons — Definition & Importance Expii How Do Electrons Form All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. They are unimaginably small, so small that. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Electrons can gain. How Do Electrons Form.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Do Electrons Form All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. They are unimaginably small, so small that. Electrons are negatively charged particles that exist in a cloud around the nucleus of an. How Do Electrons Form.
From sciencenotes.org
List of Electron Configurations of Elements How Do Electrons Form Electrons can gain enough energy to leave atomic nuclei behind. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of. How Do Electrons Form.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps How Do Electrons Form Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. These free electrons mix with ions to form a plasma. They are unimaginably small, so small that. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. Electrons are negatively charged particles that exist. How Do Electrons Form.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology How Do Electrons Form They are unimaginably small, so small that. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. In this chapter, we describe how electrons are arranged in atoms and how the spatial. How Do Electrons Form.
From courses.lumenlearning.com
3.4 Electronic Structure of Atoms (Electron Configurations) General How Do Electrons Form These free electrons mix with ions to form a plasma. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Electrons are negatively charged particles that exist in a cloud around. How Do Electrons Form.
From www.britannica.com
Electron Definition, Mass, & Facts Britannica How Do Electrons Form Electrons can gain enough energy to leave atomic nuclei behind. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. They are unimaginably small, so small that. These free electrons mix with ions to form a plasma. All atoms have the same number of electrons as protons, so the positive and negative charges. How Do Electrons Form.
From www.expii.com
Electrons — Structure & Properties Expii How Do Electrons Form Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. Electrons are negatively charged particles that exist. How Do Electrons Form.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics How Do Electrons Form They are unimaginably small, so small that. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons. How Do Electrons Form.
From www2.chemistry.msu.edu
Electron Configurations & The Periodic Table How Do Electrons Form Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Electrons can gain enough energy to leave atomic nuclei behind. These free electrons mix with ions to form a plasma. Electrons are elementary subatomic particles with. How Do Electrons Form.
From gzscienceclassonline.weebly.com
1. Electron Configuration How Do Electrons Form Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Electrons can gain enough energy to leave atomic nuclei behind. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. Electrons are negatively charged particles that exist in a cloud around the nucleus of. How Do Electrons Form.
From howtomechatronics.com
What is Electric Charge and How Electricity Works How To Mechatronics How Do Electrons Form They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. They are unimaginably small, so small that. In this chapter, we describe how electrons are arranged in atoms and how the spatial. How Do Electrons Form.
From chemistry291.blogspot.com
What Is the Electron Configuration with Step by Step Guides to write How Do Electrons Form They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Electrons can gain enough energy to leave atomic nuclei behind. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms,. How Do Electrons Form.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science How Do Electrons Form They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. They are unimaginably small, so small that. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom.. How Do Electrons Form.
From www.broadlearnings.com
Atomic Structure Broad Learnings How Do Electrons Form These free electrons mix with ions to form a plasma. They are unimaginably small, so small that. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Electrons can gain enough energy to leave atomic nuclei behind. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. Electrons. How Do Electrons Form.
From www.focuskimia.com
General Chemistry Review Valence Electrons of The First Row Elements How Do Electrons Form Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. They are unimaginably small, so small that. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related. How Do Electrons Form.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram How Do Electrons Form These free electrons mix with ions to form a plasma. They are unimaginably small, so small that. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. Electrons are elementary subatomic particles with negligible mass that surround. How Do Electrons Form.
From www.pinterest.com
What Happens When Atoms Bond infographic diagram showing how electrons How Do Electrons Form In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. Electrons can gain enough energy to leave atomic nuclei behind. These free electrons mix with ions to form a plasma. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. Electrons are. How Do Electrons Form.
From emedia.rmit.edu.au
Covalent bonds Learning Lab How Do Electrons Form Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. They are bound to. How Do Electrons Form.
From sciencenotes.org
What Is an Electron? Definition and Facts How Do Electrons Form Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. These free electrons mix with ions to. How Do Electrons Form.
From www.slideserve.com
PPT How do atoms form ions? PowerPoint Presentation, free download How Do Electrons Form Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. These free electrons mix with ions to form a plasma. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. Electrons can gain enough energy to leave atomic nuclei behind. Electrons are negatively. How Do Electrons Form.
From philschatz.com
Chemical Bonds · Anatomy and Physiology How Do Electrons Form These free electrons mix with ions to form a plasma. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Electrons can gain enough energy to leave atomic nuclei behind. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. Electrons are elementary subatomic particles. How Do Electrons Form.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation How Do Electrons Form These free electrons mix with ions to form a plasma. All atoms have the same number of electrons as protons, so the positive and negative charges cancel out,. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. Electrons. How Do Electrons Form.
From alevelchemistry.co.uk
Electron Structure ALevel Chemistry Revision Notes How Do Electrons Form Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. These free electrons mix with ions to form a plasma. Electrons can gain enough energy to leave atomic nuclei behind. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. They are unimaginably small, so small that.. How Do Electrons Form.
From spmchemistry.blog.onlinetuition.com.my
Formation of Ion SPM Chemistry How Do Electrons Form They are unimaginably small, so small that. Electron, one of the three basic subatomic particles—along with protons and neutrons—that make up atoms, the basic. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. All atoms have the same number of electrons as protons, so the positive and negative charges. How Do Electrons Form.
From www.britannica.com
Covalent bond Definition, Properties, Examples, & Facts Britannica How Do Electrons Form They are unimaginably small, so small that. They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. These free electrons mix with ions to form a plasma. Electrons can gain enough energy to leave. How Do Electrons Form.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology How Do Electrons Form They are bound to the nucleus due to electromagnetic attraction, with neutral atoms having equal numbers of protons and electrons. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. They are unimaginably small,. How Do Electrons Form.
From owlcation.com
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together How Do Electrons Form In this chapter, we describe how electrons are arranged in atoms and how the spatial arrangements of electrons are related to. Electrons are elementary subatomic particles with negligible mass that surround the nucleus of an atom. They are unimaginably small, so small that. Electrons are negatively charged particles that exist in a cloud around the nucleus of an atom. All. How Do Electrons Form.