Lab Molar Volume Of A Gas . 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted value for the. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. Mg (s) + 2 hcl (aq) h 2. according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. at standard temperature and pressure (also known as stp), viz.
from www.chegg.com
the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted value for the. revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: at standard temperature and pressure (also known as stp), viz. in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. Mg (s) + 2 hcl (aq) h 2. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure.
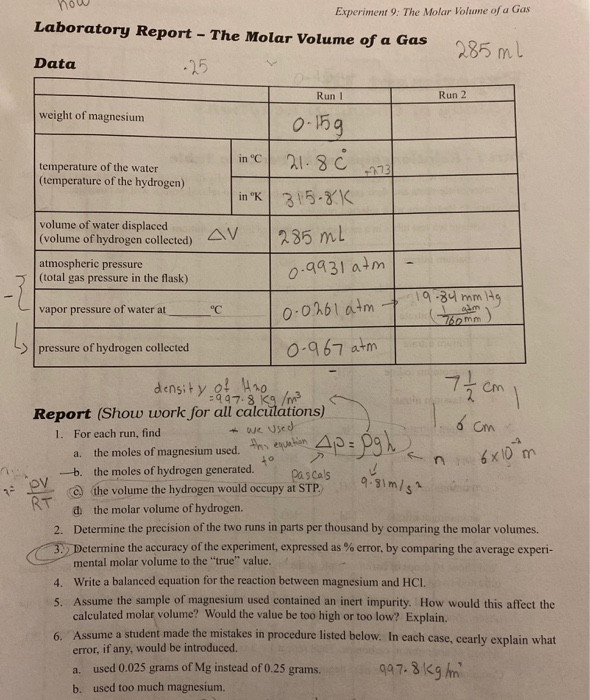
how Experiment 9 The Molar Volume of a Gas
Lab Molar Volume Of A Gas according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. at standard temperature and pressure (also known as stp), viz. 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted value for the. using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. Mg (s) + 2 hcl (aq) h 2. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k.
From www.pc.maricopa.edu
Molar Volume of a Gas Lab Molar Volume Of A Gas at standard temperature and pressure (also known as stp), viz. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts. Lab Molar Volume Of A Gas.
From www.studocu.com
Lab 4 determining molar volume of a gas Catalog No. AP Publication No Lab Molar Volume Of A Gas the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. 0°c (273.15 k) and 1 atm (101.3. Lab Molar Volume Of A Gas.
From www.studocu.com
Molar Volume of a Gas Lab b) the hydrochloric acid is the limiting Lab Molar Volume Of A Gas the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: Mg (s) + 2 hcl (aq) h 2. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. revision notes on 4.1.1. Lab Molar Volume Of A Gas.
From studylib.net
Lab 7B The Molar Volume of a Gas Lab Molar Volume Of A Gas in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the. Lab Molar Volume Of A Gas.
From www.scribd.com
Lab Experimental Determination of The Molar Volume of A Gas Revised Lab Molar Volume Of A Gas according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. in this experiment, you will be determining the molar volume. Lab Molar Volume Of A Gas.
From studylib.net
Molar Volume of a Gas at STP Lab Lab Molar Volume Of A Gas in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. the objectives of this experiment are to determine the molar volume of h 2 gas produced. Lab Molar Volume Of A Gas.
From slideplayer.com
Molar Volume of a Gas Lab ppt download Lab Molar Volume Of A Gas the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. the molar volume has the si unit of cubic metres per mole (m 3 /mol),. Lab Molar Volume Of A Gas.
From www.chegg.com
Solved The Molar Volume of a Gas In this experiment, you Lab Molar Volume Of A Gas using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted value for the. the molar. Lab Molar Volume Of A Gas.
From www.youtube.com
14The mole and the volume of gases (1st year secondary first term Lab Molar Volume Of A Gas using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. Mg (s) + 2 hcl (aq) h 2. according to avogadro’s law, the volume of one mole of any gas. Lab Molar Volume Of A Gas.
From www.youtube.com
MOLAR VOLUME OF HYDROGEN GAS LAB SETUP AND CALCULATIONS YouTube Lab Molar Volume Of A Gas revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: in this reaction, one mole of calcium carbonate makes one mole of carbon. Lab Molar Volume Of A Gas.
From studylib.net
Molar Volume of a Gas Lab Molar Volume Of A Gas at standard temperature and pressure (also known as stp), viz. using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. Mg (s) + 2 hcl (aq). Lab Molar Volume Of A Gas.
From es.scribd.com
05 Determining The Molar Volume of A Gas Molar Concentration Mole Lab Molar Volume Of A Gas according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. at standard temperature and pressure (also known as stp), viz. in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. Mg (s) + 2 hcl (aq) h 2. revision notes on. Lab Molar Volume Of A Gas.
From www.slideserve.com
PPT Lesson 2 The Molar Relationships PowerPoint Presentation, free Lab Molar Volume Of A Gas according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. Mg (s) + 2 hcl (aq) h 2. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. at standard temperature and pressure (also known as stp), viz.. Lab Molar Volume Of A Gas.
From www.studocu.com
Lab Molar Volume of a Gas Molar Volume of a Gas Experiment Teacher Lab Molar Volume Of A Gas according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it. Lab Molar Volume Of A Gas.
From www.youtube.com
MOLAR VOLUME OF A GAS PreLab NYA General Chemistry YouTube Lab Molar Volume Of A Gas in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it. Lab Molar Volume Of A Gas.
From studylib.net
LAB Molar Volume of a Gas Lab Molar Volume Of A Gas according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it. Lab Molar Volume Of A Gas.
From www.youtube.com
Molar Volume of Gases The Mole Concept YouTube Lab Molar Volume Of A Gas in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. Mg (s) + 2 hcl (aq) h 2. using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the. Lab Molar Volume Of A Gas.
From www.vernier.com
The Molar Volume of a Gas > Experiment 5 from Advanced Chemistry with Lab Molar Volume Of A Gas the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. using the ideal gas law and dalton's law, the measured volume will be. Lab Molar Volume Of A Gas.
From www.coursehero.com
[Solved] Lab molar volume determination of gas Question 1 Consider the Lab Molar Volume Of A Gas according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. in this reaction, one mole of calcium carbonate makes one mole of carbon. Lab Molar Volume Of A Gas.
From www.yumpu.com
(H) Chemistry Name Molar Volume of a gas Blk Date (Butane Lab Lab Molar Volume Of A Gas the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. in this experiment, you will be determining the molar volume of a gas at room temperature and. Lab Molar Volume Of A Gas.
From www.flinnsci.ca
Molar Volume of Hydrogen Combining the Gas Laws—ChemTopic™ Lab Lab Molar Volume Of A Gas the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. the objectives of this experiment are. Lab Molar Volume Of A Gas.
From www.scribd.com
Determining the Molar Volume of a Gas Lab Lab Molar Volume Of A Gas the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted value for the. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction:. Lab Molar Volume Of A Gas.
From chemistrymadesimple.net
Molar Volume of Gases What It Is and How To Use It Chemistry Made Lab Molar Volume Of A Gas Mg (s) + 2 hcl (aq) h 2. in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted value. Lab Molar Volume Of A Gas.
From studylib.net
The Molar Volume of a Gas and R Lab Lab Molar Volume Of A Gas at standard temperature and pressure (also known as stp), viz. 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted value for the. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: according to avogadro’s law, the volume of one mole of any gas at standard temperature. Lab Molar Volume Of A Gas.
From www.chegg.com
how Experiment 9 The Molar Volume of a Gas Lab Molar Volume Of A Gas Mg (s) + 2 hcl (aq) h 2. at standard temperature and pressure (also known as stp), viz. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. according to avogadro’s law, the volume of one mole of any gas at. Lab Molar Volume Of A Gas.
From www.slideserve.com
PPT Molar Volume of a Gas PowerPoint Presentation, free download ID Lab Molar Volume Of A Gas revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus, written by the chemistry experts at save my exams. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: the molar volume has the si unit of cubic metres per mole (m. Lab Molar Volume Of A Gas.
From www.youtube.com
Ideal Gas Equation Molar Volume at Standard Pressure and Temperature Lab Molar Volume Of A Gas in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. Mg (s) + 2 hcl (aq) h 2. the objectives of this experiment are to determine the molar volume of h 2. Lab Molar Volume Of A Gas.
From www.youtube.com
Molar Volume of a Gas Lab Part 1 YouTube Lab Molar Volume Of A Gas according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. at standard temperature and pressure (also known as stp), viz. Mg (s) + 2 hcl (aq) h 2.. Lab Molar Volume Of A Gas.
From www.slideserve.com
PPT Lesson 2 The Molar Relationships PowerPoint Presentation, free Lab Molar Volume Of A Gas at standard temperature and pressure (also known as stp), viz. Mg (s) + 2 hcl (aq) h 2. in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. the objectives of this experiment are. Lab Molar Volume Of A Gas.
From www.chegg.com
1 Molar Volume of an Ideal Gas_Essay1_Lab Results Lab Molar Volume Of A Gas using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. the molar volume has the si unit of cubic metres per mole (m. Lab Molar Volume Of A Gas.
From studylib.net
The Molar Volume of a Gas Lab Molar Volume Of A Gas in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted value for the. according to avogadro’s law, the volume of one mole of any gas at standard temperature and pressure (stp = 273 k. the molar volume has the si unit of cubic. Lab Molar Volume Of A Gas.
From www.studypool.com
SOLUTION Experiment of Molar Volume of a Gas at STP Lab Report Studypool Lab Molar Volume Of A Gas in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: Mg (s) + 2 hcl (aq) h 2. revision notes on 4.1.1 molar volume of a gas for the edexcel a level chemistry syllabus,. Lab Molar Volume Of A Gas.
From www.youtube.com
Molar Volume of a Gas AP Chemistry YouTube Lab Molar Volume Of A Gas the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. Mg (s) + 2 hcl (aq) h 2. in this reaction, one mole of calcium carbonate makes one mole of carbon dioxide. 0°c (273.15 k) and 1 atm (101.3 kpa), the accepted. Lab Molar Volume Of A Gas.
From myans.bhantedhammika.net
Molar Volume Of A Gas Lab Magnesium + Hydrochloric Acid Lab Molar Volume Of A Gas using the ideal gas law and dalton's law, the measured volume will be corrected to obtain the volume of dry hydrogen at room. the molar volume has the si unit of cubic metres per mole (m 3 /mol), [1] although it is more typical to use the units cubic. revision notes on 4.1.1 molar volume of a. Lab Molar Volume Of A Gas.
From www.youtube.com
Molar Volume of Gases Examples The Mole Concept YouTube Lab Molar Volume Of A Gas the objectives of this experiment are to determine the molar volume of h 2 gas produced by the reaction: in this experiment, you will be determining the molar volume of a gas at room temperature and pressure. Mg (s) + 2 hcl (aq) h 2. according to avogadro’s law, the volume of one mole of any gas. Lab Molar Volume Of A Gas.