Standard Cell Potential Given . The potential difference is caused by the ability of electrons to flow. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Cell potential, free energy, and equilibrium constant. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. Describe and relate the definitions of electrode and cell potentials.
from www.youtube.com
The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential difference is caused by the ability of electrons to flow. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Cell potential, free energy, and equilibrium constant.
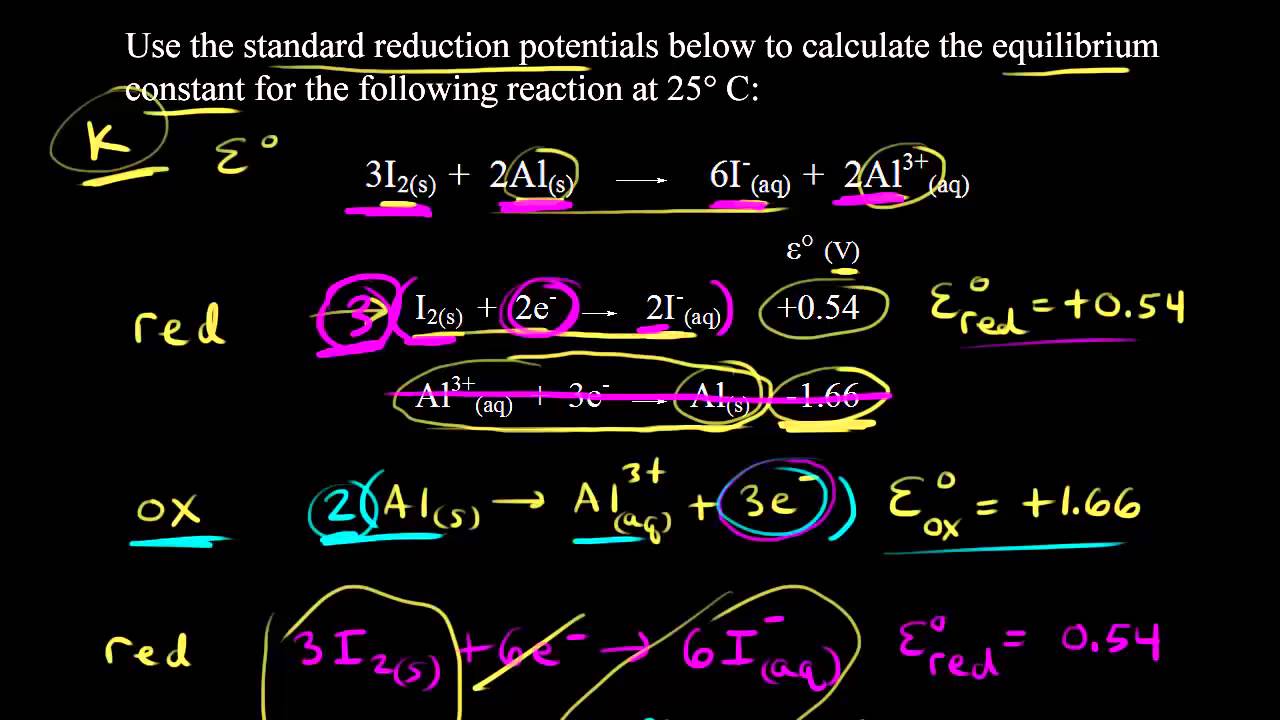
Calculating the equilibrium constant from the standard cell potential
Standard Cell Potential Given We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Describe and relate the definitions of electrode and cell potentials. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Cell potential, free energy, and equilibrium constant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. The potential difference is caused by the ability of electrons to flow. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Interpret electrode potentials in terms of relative oxidant and reductant. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods:
From www.bartleby.com
Answered 31)Calculate the cell potential for the… bartleby Standard Cell Potential Given The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Cell potential, free energy, and equilibrium constant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential difference is caused. Standard Cell Potential Given.
From www.youtube.com
Electrochemistry 07 Calculating Nonstandard Cell Potentials YouTube Standard Cell Potential Given The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. Cell potential, free energy, and equilibrium constant. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined. Standard Cell Potential Given.
From askfilo.com
20 Standard cell potential for given cell is Zn∣∣ Zn2+(0.01M)∥Cu2+(0.1M)∣.. Standard Cell Potential Given Cell potential, free energy, and equilibrium constant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials. Standard Cell Potential Given.
From www.youtube.com
How to Calculate Standard Cell Potential and Voltage Part 1 Examples Standard Cell Potential Given The potential difference is caused by the ability of electrons to flow. Cell potential, free energy, and equilibrium constant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell. Standard Cell Potential Given.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID7041672 Standard Cell Potential Given The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. Cell potential, free energy, and equilibrium constant. Describe and relate the definitions. Standard Cell Potential Given.
From mungfali.com
Nernst Equation Cell Potential Standard Cell Potential Given The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Describe and relate the definitions of electrode and cell potentials. Cell potential, free energy, and equilibrium constant. The standard cell potential (e°cell) is measured at 298 k and all. Standard Cell Potential Given.
From askfilo.com
18 2/20100 Standard cell potential for given cell is Zn∣Zn2+(0.01M)Cu2+(0.. Standard Cell Potential Given Cell potential, free energy, and equilibrium constant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential of the cell under standard. Standard Cell Potential Given.
From www.youtube.com
Calculating the Cell Potential of Electrochemical Cells. (Adv Chem Ch Standard Cell Potential Given Cell potential, free energy, and equilibrium constant. Describe and relate the definitions of electrode and cell potentials. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases,. Standard Cell Potential Given.
From www.numerade.com
SOLVED Calculate the standard cell potential given the following Standard Cell Potential Given The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. Cell potential,. Standard Cell Potential Given.
From www.numerade.com
SOLVED Cell Potential 8 of 30 Review Constants Periodic Table For a Standard Cell Potential Given Cell potential, free energy, and equilibrium constant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. This is. Standard Cell Potential Given.
From www.chegg.com
Solved Using the Standard Cell Potential Equation, Ecell = Standard Cell Potential Given The potential difference is caused by the ability of electrons to flow. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Cell potential, free energy, and equilibrium constant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and. Standard Cell Potential Given.
From scienceinfo.com
Cell Potential & Standard Cell Potential Standard Cell Potential Given This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The cell potential, \(e_{cell}\),. Standard Cell Potential Given.
From www.tpsearchtool.com
Standard Reduction Potential Charts For Chemistry Images Standard Cell Potential Given We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. The potential difference is caused by. Standard Cell Potential Given.
From www.youtube.com
Calculating the equilibrium constant from the standard cell potential Standard Cell Potential Given This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Interpret electrode potentials in terms of relative oxidant and reductant. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. The standard cell potential (e°cell) is measured at 298 k and. Standard Cell Potential Given.
From www.youtube.com
STANDARD CELL POTENTIAL YouTube Standard Cell Potential Given We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. This is also known as the standard cell potential (e cell ꝋ). Standard Cell Potential Given.
From www.youtube.com
2039 Calculating Standard Cell Potentials YouTube Standard Cell Potential Given Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. This is also known as. Standard Cell Potential Given.
From www.chegg.com
Solved 17. Using standard reduction potentials (on the next Standard Cell Potential Given The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Cell potential, free energy, and equilibrium constant. We have seen, in this chapter, that a positive standard cell potential. Standard Cell Potential Given.
From www.chegg.com
Solved Use the halfreactions below to produce a voltaic Standard Cell Potential Given Cell potential, free energy, and equilibrium constant. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. Interpret electrode potentials in terms of relative oxidant and reductant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard. Standard Cell Potential Given.
From www.chegg.com
Solved C. Calculating Standard Cell Potentials, Eºcell Given Standard Cell Potential Given Cell potential, free energy, and equilibrium constant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical.. Standard Cell Potential Given.
From www.youtube.com
Emf of cell,Cell potential (Electrochemistry part 13 for CBSE class 12 Standard Cell Potential Given The potential difference is caused by the ability of electrons to flow. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Cell potential, free energy, and equilibrium constant. The cell. Standard Cell Potential Given.
From salma-has-blevins.blogspot.com
How to Calculate Cell Potential Under Standard Conditions Salmahas Standard Cell Potential Given The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. Describe and. Standard Cell Potential Given.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Standard Cell Potential Given The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential difference is caused by the. Standard Cell Potential Given.
From askfilo.com
20 Review Standard cell potential for given cell is Zn∣∣ Zn2+(0.01 M) )∥C.. Standard Cell Potential Given The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The standard cell potential (e°cell) is measured at 298 k. Standard Cell Potential Given.
From general.chemistrysteps.com
How to Calculate Standard Cell Potential Chemistry Steps Standard Cell Potential Given The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The potential difference is caused by the ability of electrons to flow. Cell potential, free energy, and equilibrium constant. This is also known as the standard cell potential (e. Standard Cell Potential Given.
From www.youtube.com
19.1 Calculating cell potential (HL) YouTube Standard Cell Potential Given We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Describe and relate the definitions of electrode and cell potentials. The potential of the cell under standard conditions (1 m for. Standard Cell Potential Given.
From www.nagwa.com
Question Video Calculating the Standard Reduction Potential of an Standard Cell Potential Given The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Describe and relate the definitions. Standard Cell Potential Given.
From www.youtube.com
Find the cell potential of a galvanic cell based on the following Standard Cell Potential Given The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: The potential difference is caused by the ability of electrons to flow. Interpret electrode potentials in terms of relative oxidant and reductant. Describe and relate the definitions of electrode and cell potentials. This is also known as the standard cell potential (e cell. Standard Cell Potential Given.
From halleldmoses.blogspot.com
Cell Potential Formula HalleldMoses Standard Cell Potential Given 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: The potential of the cell under standard. Standard Cell Potential Given.
From kunduz.com
[ANSWERED] a given galvanic cell the standard cell potential be Kunduz Standard Cell Potential Given The potential difference is caused by the ability of electrons to flow. Describe and relate the definitions of electrode and cell potentials. Interpret electrode potentials in terms of relative oxidant and reductant. Cell potential, free energy, and equilibrium constant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. The potential. Standard Cell Potential Given.
From www.nagwa.com
Question Video Calculating a Standard Cell Potential from Standard Standard Cell Potential Given The potential difference is caused by the ability of electrons to flow. Cell potential, free energy, and equilibrium constant. 1 atm for gases, 1 m for solutions, and the pure solid for electrodes. Interpret electrode potentials in terms of relative oxidant and reductant. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure. Standard Cell Potential Given.
From askfilo.com
21 ω^ Review Standard cell potential for given cell is Zn∣∣ Zn2+(0.01 M ).. Standard Cell Potential Given We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. The potential of the cell under standard conditions (1 m for solutions, 1 atm for gases, pure solids or liquids for other substances) and at a fixed temperature (25°c) is called. The potential difference is caused by the ability of electrons to flow.. Standard Cell Potential Given.
From studymarxianism.z21.web.core.windows.net
How To Calculate E Cell Potential Standard Cell Potential Given Cell potential, free energy, and equilibrium constant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. Interpret electrode potentials in terms of relative oxidant and reductant. The potential difference is caused by the ability of electrons to flow. This is also known as the standard cell potential (e cell ꝋ). Standard Cell Potential Given.
From www.coursehero.com
[Solved] 24. Calculate the standard cell potential given the following Standard Cell Potential Given Interpret electrode potentials in terms of relative oxidant and reductant. The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: Cell potential, free energy, and equilibrium constant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard. Standard Cell Potential Given.
From www.pinterest.co.kr
Standard Reduction Potential (E) when given two half reactions and Standard Cell Potential Given The standard cell potential (e°cell) is measured at 298 k and all components in their standard states: We have seen, in this chapter, that a positive standard cell potential (e°cell) indicates a spontaneous electrochemical. Cell potential, free energy, and equilibrium constant. Describe and relate the definitions of electrode and cell potentials. The potential difference is caused by the ability of. Standard Cell Potential Given.
From www.chegg.com
Solved Given the following reduction potentials, what is the Standard Cell Potential Given Interpret electrode potentials in terms of relative oxidant and reductant. The cell potential, \(e_{cell}\), is the measure of the potential difference between two half cells in an electrochemical cell. This is also known as the standard cell potential (e cell ꝋ) the standard cell potential can be determined by two methods: Cell potential, free energy, and equilibrium constant. 1 atm. Standard Cell Potential Given.