Fda Labeling Changes Guidance . The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective.
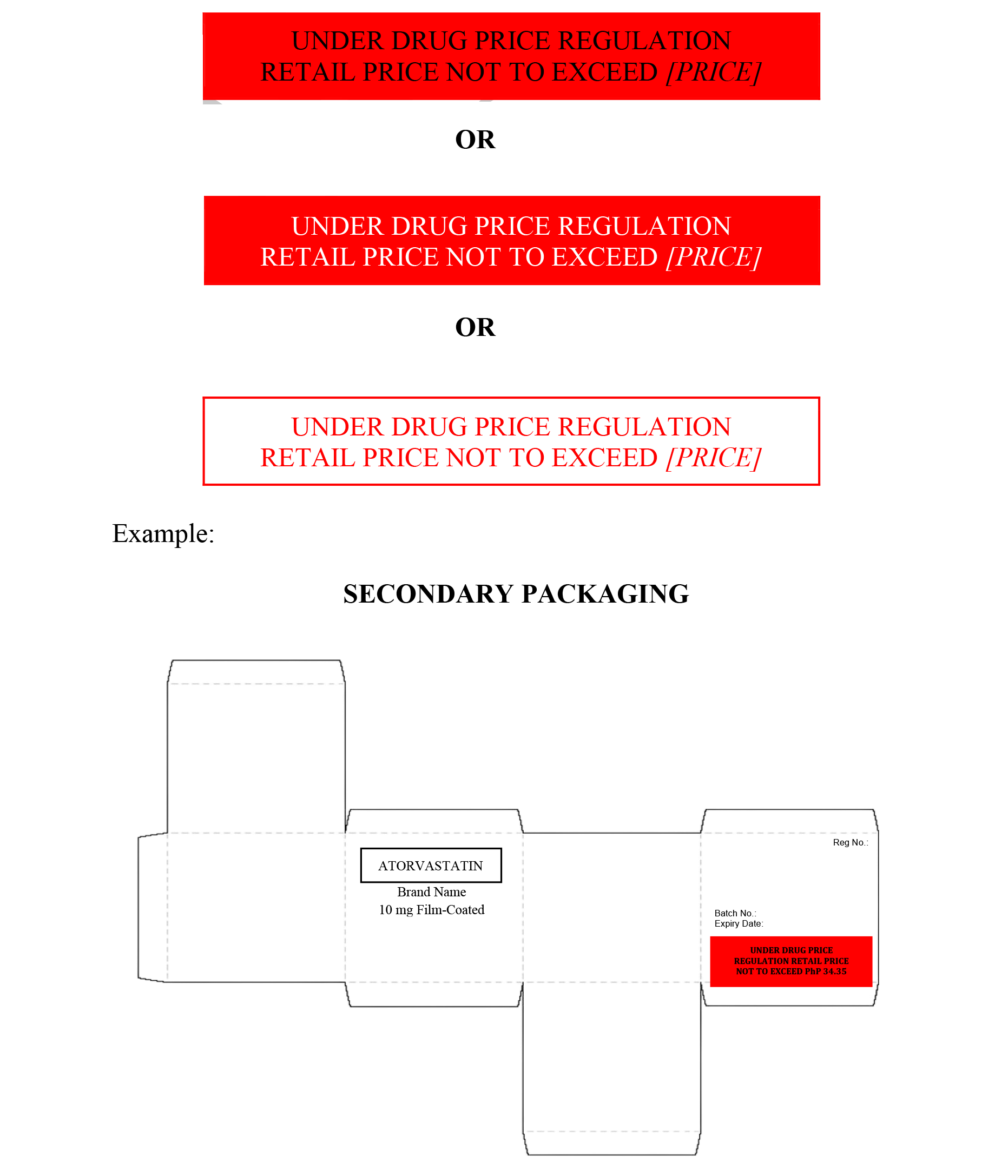
from www.fda.gov.ph
This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective.
Draft for Comments Guidelines on Labeling Requirements of Drug
Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Labeling Changes Guidance Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Human prescription drug labeling. Fda Labeling Changes Guidance.
From www.medscape.com
FDA Safety Labeling Changes AprilJune 2017 Page 4 Fda Labeling Changes Guidance As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This draft guidance provides recommendations to applicants of approved new drug. Fda Labeling Changes Guidance.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. As part of fda’s medwatch program, important. Fda Labeling Changes Guidance.
From www.slideserve.com
PPT medical device labeling regulation Changes by FDA for covid 19 Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). This draft guidance provides recommendations to applicants of approved new. Fda Labeling Changes Guidance.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Labeling Changes Guidance Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Ensure labeling meets statutory/regulatory requirements and is. Fda Labeling Changes Guidance.
From bodynsoil.com
FDA Making Food Labels Easier to Read and Understand BodynSoil Fda Labeling Changes Guidance Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). This. Fda Labeling Changes Guidance.
From www.scribd.com
FDA Cosmetic Labeling Guide PDF Fda Labeling Changes Guidance This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Human prescription. Fda Labeling Changes Guidance.
From www.fdalisting.com
U.S. FDA Food, Beverage and Dietary Supplement Labeling Requirements Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. As part. Fda Labeling Changes Guidance.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Labeling Changes Guidance Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including.. Fda Labeling Changes Guidance.
From www.nfpt.com
Understanding the Changes to the 2018 FDA Nutrition Label Fda Labeling Changes Guidance This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. This guidance provides recommendations for updating labeling for abbreviated new. Fda Labeling Changes Guidance.
From www.bevnet.com
FDA Unveils Updates to Nutrition Facts Panel Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). This draft guidance provides recommendations to applicants of approved new drug applications (ndas). Fda Labeling Changes Guidance.
From thefoodsafetyfoundation.locals.com
Shared post Allergen Labeling Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This guidance provides recommendations for. Fda Labeling Changes Guidance.
From www.vrogue.co
Food Labeling 101 Fda Regulations Guide 2022 Artwork vrogue.co Fda Labeling Changes Guidance Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. This draft guidance provides recommendations. Fda Labeling Changes Guidance.
From blog.globalvision.co
Your Complete Guide to Meeting FDA Labeling Requirements Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Human prescription drug labeling (1) contains a summary of the essential scientific information. Fda Labeling Changes Guidance.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Labeling Changes Guidance Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). The food and drug. Fda Labeling Changes Guidance.
From packaginghub.com
FDA Labeling Requirements For Cosmetics Packaging Hub Fda Labeling Changes Guidance As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This guidance provides recommendations for. Fda Labeling Changes Guidance.
From rasladeloris.pages.dev
Fda Food Labeling Guide 2025 Pauli Bethanne Fda Labeling Changes Guidance As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. Ensure labeling meets statutory/regulatory requirements and is consistent with final. Fda Labeling Changes Guidance.
From animalia-life.club
Fda Drug Labeling Requirements Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Ensure labeling meets statutory/regulatory requirements. Fda Labeling Changes Guidance.
From www.fda.gov
Guidance for Industry Nutrition Labeling Manual A Guide for Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. As part of fda’s medwatch program, important changes to the safety labeling. Fda Labeling Changes Guidance.
From gioljoiba.blob.core.windows.net
Fda Labeling Font Size at Anthony Schreiber blog Fda Labeling Changes Guidance Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Human prescription drug labeling (1) contains a summary of the essential scientific information. Fda Labeling Changes Guidance.
From blog.alchemysystems.com
FDA Labeling Changes A Survival Guide The Feed Fda Labeling Changes Guidance Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Human prescription drug labeling (1) contains a summary. Fda Labeling Changes Guidance.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Labeling Changes Guidance Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe. Fda Labeling Changes Guidance.
From www.celegence.com
FDA Issues Guidance for Industry Biosimilar Labeling Celegence Fda Labeling Changes Guidance Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. As part of fda’s medwatch program, important changes to the safety labeling of drugs. Fda Labeling Changes Guidance.
From ar.inspiredpencil.com
Fda Labeling Requirements For Food Fda Labeling Changes Guidance Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Ensure labeling meets statutory/regulatory requirements and is consistent. Fda Labeling Changes Guidance.
From ac864f81ed.nxcli.net
Food Packaging Design FDA Labeling Guidelines 2020 Deal Design Fda Labeling Changes Guidance As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Safety labeling changes — implementation of section 505(o)(4). Fda Labeling Changes Guidance.
From ar.inspiredpencil.com
Fda Labeling Requirements For Food Fda Labeling Changes Guidance As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This guidance provides recommendations for. Fda Labeling Changes Guidance.
From ar.inspiredpencil.com
Fda Labeling Regulations Fda Labeling Changes Guidance This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Ensure labeling meets statutory/regulatory requirements and is consistent with final. Fda Labeling Changes Guidance.
From www.celegence.com
FDA Guidance on Navigating Annual Reportable Labeling Changes Fda Labeling Changes Guidance As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Human prescription drug labeling (1) contains a summary of the essential scientific information needed for. Fda Labeling Changes Guidance.
From www.lachmanconsultants.com
Guidance on Labeling Changes for Approved OTC NDAs/ANDAs Provides AR Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic. Fda Labeling Changes Guidance.
From ar.inspiredpencil.com
Fda Labeling Requirements For Food Fda Labeling Changes Guidance This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for. Fda Labeling Changes Guidance.
From www.linkedin.com
FDA Issues Labelling Guidance Fda Labeling Changes Guidance Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Human prescription drug labeling. Fda Labeling Changes Guidance.
From www.vrogue.co
Modifications To Fda Food Labeling Requirements Tempo vrogue.co Fda Labeling Changes Guidance This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. Human prescription. Fda Labeling Changes Guidance.
From www.foodsafetyselect.com
U.S. FDA’s 2024 Draft Guidance on Food Labeling Overview & Insights Fda Labeling Changes Guidance The food and drug administration (fda or agency) is announcing the availability of a draft guidance for industry. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. Human prescription. Fda Labeling Changes Guidance.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Labeling Changes Guidance As part of fda’s medwatch program, important changes to the safety labeling of drugs and therapeutic biologicals, including. This draft guidance provides recommendations to applicants of approved new drug applications (ndas) and abbreviated new. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug,. Fda Labeling Changes Guidance.
From www.fda.gov.ph
Draft for Comments Guidelines on Labeling Requirements of Drug Fda Labeling Changes Guidance Human prescription drug labeling (1) contains a summary of the essential scientific information needed for the safe and effective. Safety labeling changes — implementation of section 505(o)(4) of the federal food, drug, and cosmetic act. This guidance provides recommendations for updating labeling for abbreviated new drug applications (andas). Ensure labeling meets statutory/regulatory requirements and is consistent with final guidance. The. Fda Labeling Changes Guidance.