Does Graphite React With Acid . Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Metals above hydrogen in the reactivity series react with acids; Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Other halogens do not appear to react with graphite. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Those below hydrogen in the reactivity series don't. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid.
from ppt-online.org
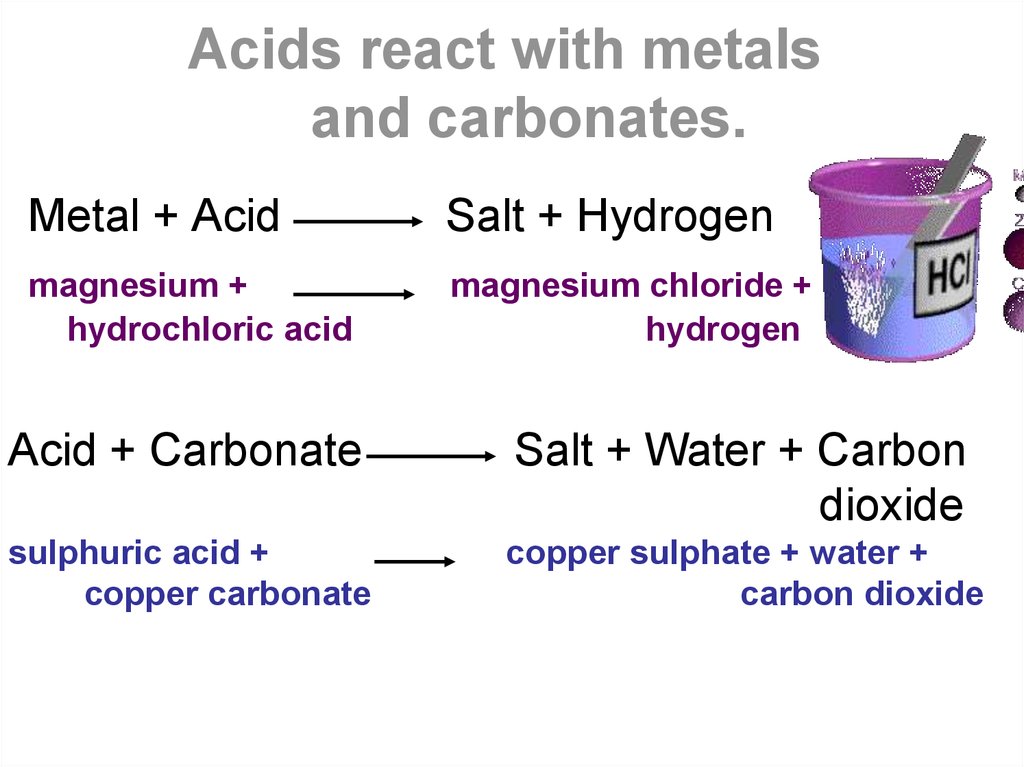
Metals above hydrogen in the reactivity series react with acids; Those below hydrogen in the reactivity series don't. Other halogens do not appear to react with graphite. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents.
Acids and alkalis презентация онлайн
Does Graphite React With Acid Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Those below hydrogen in the reactivity series don't. Other halogens do not appear to react with graphite. Metals above hydrogen in the reactivity series react with acids; Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Aside from hcl, there are other substances that can potentially react with graphite under certain conditions.
From pubs.acs.org
Revisiting the Oxidation of Graphite Reaction Mechanism, Chemical Stability, and Structure Self Does Graphite React With Acid Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Other halogens do not appear to react with graphite. Metals above hydrogen in the reactivity series react with acids; Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both. Does Graphite React With Acid.
From www.researchgate.net
Schematic illustration of the graphite oxidation mechanism. Big dark... Download Scientific Does Graphite React With Acid Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Other halogens do not appear to react with graphite. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Heavier. Does Graphite React With Acid.
From pubs.acs.org
Revisiting the Oxidation of Graphite Reaction Mechanism, Chemical Stability, and Structure Self Does Graphite React With Acid Metals above hydrogen in the reactivity series react with acids; Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Through a process known as. Does Graphite React With Acid.
From www.youtube.com
How acids react with metal carbonates CBSE Class 10 Chemistry Notes YouTube Does Graphite React With Acid Other halogens do not appear to react with graphite. Metals above hydrogen in the reactivity series react with acids; Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Hydrochloric acid, sulfuric acid,. Does Graphite React With Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Does Graphite React With Acid Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Other halogens do not appear to react with graphite. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the. Does Graphite React With Acid.
From ppt-online.org
Acids and alkalis презентация онлайн Does Graphite React With Acid Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Other halogens do not appear to react with graphite. Hydrochloric acid, sulfuric acid,. Does Graphite React With Acid.
From www.researchgate.net
Schematics of the chemical reactions between graphite, GO, IL... Download Scientific Diagram Does Graphite React With Acid Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and.. Does Graphite React With Acid.
From pubs.acs.org
Revisiting the Oxidation of Graphite Reaction Mechanism, Chemical Stability, and Structure Self Does Graphite React With Acid Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Other halogens do not appear to react with graphite. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Those below hydrogen in the reactivity. Does Graphite React With Acid.
From pubs.rsc.org
Graphite sulphate a precursor to graphene Chemical Communications (RSC Publishing) DOI10. Does Graphite React With Acid Those below hydrogen in the reactivity series don't. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Metals above hydrogen in the. Does Graphite React With Acid.
From www.researchgate.net
The reaction mechanism of sulfuric acid with polyethylene, introducing... Download Scientific Does Graphite React With Acid Other halogens do not appear to react with graphite. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Metals above hydrogen in the reactivity series react with acids; Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid.. Does Graphite React With Acid.
From www.mdpi.com
Catalysts Free FullText Gaseous Nitric Acid Activated Graphite Felts as Hierarchical Metal Does Graphite React With Acid Other halogens do not appear to react with graphite. Metals above hydrogen in the reactivity series react with acids; Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the. Does Graphite React With Acid.
From mavink.com
Acid React With Base Does Graphite React With Acid Metals above hydrogen in the reactivity series react with acids; Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Mellitic acid, c. Does Graphite React With Acid.
From chem.libretexts.org
6.7 Boron Oxides, Hydroxides, and Oxyanions Chemistry LibreTexts Does Graphite React With Acid Those below hydrogen in the reactivity series don't. Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the. Does Graphite React With Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Does Graphite React With Acid Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Heavier alkali metals. Does Graphite React With Acid.
From revisechemistry.uk
Reactivity of Metals AQA C4 revisechemistry.uk Does Graphite React With Acid Other halogens do not appear to react with graphite. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Through a process known as hydrolysis, the ions. Does Graphite React With Acid.
From utedzz.blogspot.com
Periodic Table Reactivity Series Of Metals Periodic Table Timeline Does Graphite React With Acid Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Those below hydrogen in the reactivity series don't. Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Heavier alkali metals react with graphite to form graphite. Does Graphite React With Acid.
From www.goodscience.com.au
AcidMetal Reactions Good Science Does Graphite React With Acid Metals above hydrogen in the reactivity series react with acids; Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Those below hydrogen in the reactivity series don't. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of. Does Graphite React With Acid.
From www.sliderbase.com
Properties of Acids Bases Presentation Chemistry Does Graphite React With Acid Other halogens do not appear to react with graphite. Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds. Does Graphite React With Acid.
From www.chegg.com
Solved Metal carbonates react with acid to produce a salt, Does Graphite React With Acid Metals above hydrogen in the reactivity series react with acids; Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water.. Does Graphite React With Acid.
From www.chegg.com
Solved Sodium bicarbonate reacts with carboxylic acids to Does Graphite React With Acid Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5. Does Graphite React With Acid.
From flatworldknowledge.lardbucket.org
Chemical Does Graphite React With Acid Other halogens do not appear to react with graphite. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Reactions of graphite with oxidizing acids or. Does Graphite React With Acid.
From www.chegg.com
Solved Metal carbonates react with acid to produce a salt, Does Graphite React With Acid Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Those below hydrogen in the reactivity series don't. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing. Does Graphite React With Acid.
From www.researchgate.net
Charging and discharging of graphite. (a) Reaction equation for the... Download Scientific Does Graphite React With Acid Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Metals above hydrogen in the reactivity series react with acids; Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Mellitic acid, c 6 (co 2. Does Graphite React With Acid.
From www.teachoo.com
[MCQ] Which of the statements is not correct? All metal oxides react Does Graphite React With Acid Those below hydrogen in the reactivity series don't. Other halogens do not appear to react with graphite. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Aside from hcl, there are other substances. Does Graphite React With Acid.
From pubs.acs.org
Revisiting the Oxidation of Graphite Reaction Mechanism, Chemical Stability, and Structure Self Does Graphite React With Acid Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Metals above hydrogen in the reactivity series react with acids; Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral. Does Graphite React With Acid.
From www.teachoo.com
Metal compound A reacts with dilute hydrochloric acid to produce Does Graphite React With Acid Metals above hydrogen in the reactivity series react with acids; Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Those below hydrogen in the reactivity series don't. Other halogens. Does Graphite React With Acid.
From www.yaclass.in
How do metal carbonates and metal hydrogencarbonates react with acids — lesson. Science CBSE Does Graphite React With Acid Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Metals above hydrogen in the reactivity series react with acids; Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Other halogens do not appear to. Does Graphite React With Acid.
From mrcartlidgesaigon.edublogs.org
C10 Metals Science) Mr Cartlidge's second Science Blog Does Graphite React With Acid Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Other halogens do not appear to react with graphite. Those below hydrogen in the reactivity series don't. Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Through a process known as hydrolysis, the ions produced when. Does Graphite React With Acid.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Chemistry Does Graphite React With Acid Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Metals above hydrogen in the reactivity series react with acids; Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Other halogens do not appear to react with graphite. Reactions. Does Graphite React With Acid.
From washedupcelebrity.blogspot.com
Draw A Structure For The Product Of The Acidbase Reaction Below The Expert Does Graphite React With Acid Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Those below hydrogen in the reactivity series don't. Through a process known as hydrolysis, the ions produced when an acid and base combine may react with the water. Aside from hcl, there are other substances that can potentially react. Does Graphite React With Acid.
From timescavengers.blog
Ocean Chemistry & Acidification Time Scavengers Does Graphite React With Acid Aside from hcl, there are other substances that can potentially react with graphite under certain conditions. Metals above hydrogen in the reactivity series react with acids; Those below hydrogen in the reactivity series don't. Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Through a process known as. Does Graphite React With Acid.
From www.mdpi.com
Metals Free FullText Purification of Waste Graphite from Crucibles Used in Photovoltaic Does Graphite React With Acid Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Metals above hydrogen in the reactivity series react with acids; Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral. Does Graphite React With Acid.
From www.teachoo.com
Reactions of Acids and Bases Full list (with Examples) Teachoo Does Graphite React With Acid Hydrochloric acid, sulfuric acid, and hydrofluoric acid) and solvents. Metals above hydrogen in the reactivity series react with acids; Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result in intercalation compounds that contain both neutral and. Other halogens do not appear to react with graphite. Through a process known as. Does Graphite React With Acid.
From www.researchgate.net
Labelled FTIR spectra of acidextracted graphite from those attached... Download Scientific Does Graphite React With Acid Metals above hydrogen in the reactivity series react with acids; Mellitic acid, c 6 (co 2 h) 6, is formed when graphite combines with the oxidizing acid hot concentrated nitric acid. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Those below hydrogen in the. Does Graphite React With Acid.
From owlcation.com
Chemical Weathering A Great Natural Force Owlcation Does Graphite React With Acid Other halogens do not appear to react with graphite. Heavier alkali metals react with graphite to form graphite intercalation compounds, substances in which metal atoms are inserted between the sheets of carbon atoms. Those below hydrogen in the reactivity series don't. Reactions of graphite with oxidizing acids or molecular oxidants such as br 2, asf 5 or fecl 3 result. Does Graphite React With Acid.