Important Buffer In Human Blood . Buffer systems in the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. In this system, gaseous metabolic waste carbon dioxide reacts. It takes only seconds for the chemical. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. It takes only seconds for the. If blood ph goes above or below that range, it can destroy cells. 3) are much greater than. This buffer works well because concentrations of the buffer components hco.
from www.numerade.com
The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. It takes only seconds for the chemical. The buffer systems in the human body are extremely efficient, and different systems work at different rates. If blood ph goes above or below that range, it can destroy cells. Buffer systems in the body. It takes only seconds for the. The buffer systems in the human body are extremely efficient, and different systems work at different rates. 3) are much greater than. In this system, gaseous metabolic waste carbon dioxide reacts.
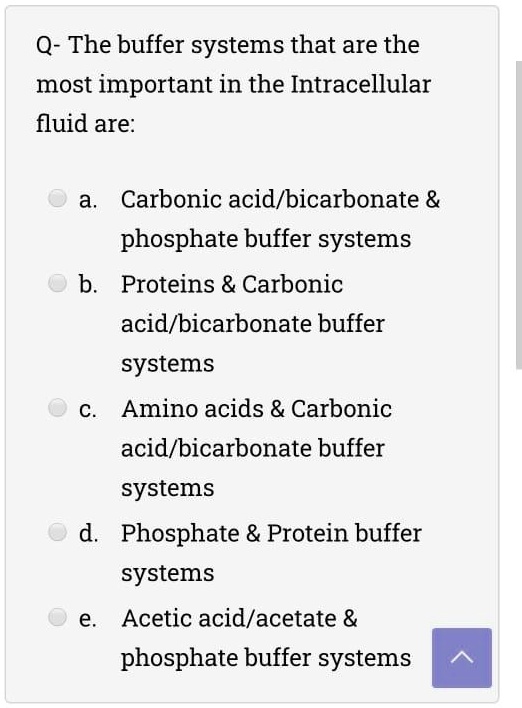
SOLVED Q The buffer systems that are the most important in the
Important Buffer In Human Blood The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. 3) are much greater than. In this system, gaseous metabolic waste carbon dioxide reacts. It takes only seconds for the chemical. It takes only seconds for the. In this system, gaseous metabolic waste carbon dioxide reacts. This buffer works well because concentrations of the buffer components hco. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. If blood ph goes above or below that range, it can destroy cells. Buffer systems in the body. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Important Buffer In Human Blood This buffer works well because concentrations of the buffer components hco. The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph.. Important Buffer In Human Blood.
From bryont.net
Human Red Blood Cell Lysis Buffer Recipe Bryont Blog Important Buffer In Human Blood This buffer works well because concentrations of the buffer components hco. It takes only seconds for the chemical. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Buffer systems in the body. The ph. Important Buffer In Human Blood.
From www.slideserve.com
PPT RESPIRATORY SYSTEM 3 PowerPoint Presentation, free download Important Buffer In Human Blood 3) are much greater than. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for. Important Buffer In Human Blood.
From www.youtube.com
Buffer action in the blood YouTube Important Buffer In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. If blood ph goes above or below that range, it can destroy cells. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Human blood contains a buffer of carbonic acid (h2co3 h 2 co. Important Buffer In Human Blood.
From pressbooks.bccampus.ca
26.3 Electrolyte Balance Douglas College Human Anatomy and Physiology Important Buffer In Human Blood The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The ph of the blood is maintained between. Important Buffer In Human Blood.
From www.youtube.com
Chemical Buffers protein buffer, phosphate buffer system and Important Buffer In Human Blood 3) are much greater than. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The buffer systems in the human body are extremely efficient, and different systems work at different rates. If blood ph goes above or below that range, it can destroy cells. This buffer works well because concentrations of the. Important Buffer In Human Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Important Buffer In Human Blood The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. In this system, gaseous metabolic waste carbon dioxide reacts. It takes only seconds for the chemical. 3) are much greater than. In this system, gaseous metabolic waste carbon dioxide reacts. This buffer works well because concentrations of the buffer components hco. The buffer systems. Important Buffer In Human Blood.
From en.ppt-online.org
Blood biochemistry online presentation Important Buffer In Human Blood This buffer works well because concentrations of the buffer components hco. It takes only seconds for the chemical. 3) are much greater than. It takes only seconds for the. If blood ph goes above or below that range, it can destroy cells. In this system, gaseous metabolic waste carbon dioxide reacts. In this system, gaseous metabolic waste carbon dioxide reacts.. Important Buffer In Human Blood.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 Important Buffer In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. If blood ph goes above or below that range, it can destroy cells. It takes only seconds for the. The buffer systems in the human body are extremely efficient, and different systems work at. Important Buffer In Human Blood.
From iastate.pressbooks.pub
Properties of Blood as a Buffer and Blood Glucose A Mixed Course Important Buffer In Human Blood If blood ph goes above or below that range, it can destroy cells. It takes only seconds for the chemical. This buffer works well because concentrations of the buffer components hco. Buffer systems in the body. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. The buffer systems in the human body are. Important Buffer In Human Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Important Buffer In Human Blood It takes only seconds for the chemical. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems in the human body are extremely efficient, and different systems work at different rates. The buffer systems in the human body are extremely efficient, and different systems work at different rates. This buffer works well because concentrations of the buffer components. Important Buffer In Human Blood.
From www.slideserve.com
PPT MLAB 2401 Clinical Chemistry Keri BrophyMartinez PowerPoint Important Buffer In Human Blood The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. This buffer works well because concentrations of the. Important Buffer In Human Blood.
From www.slideshare.net
Blood buffer system PPT Important Buffer In Human Blood It takes only seconds for the chemical. 3) are much greater than. It takes only seconds for the. The buffer systems in the human body are extremely efficient, and different systems work at different rates. If blood ph goes above or below that range, it can destroy cells. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains. Important Buffer In Human Blood.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Important Buffer In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. Buffer systems in the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the. The ph of the blood is. Important Buffer In Human Blood.
From www.slideshare.net
Buffer system Important Buffer In Human Blood It takes only seconds for the chemical. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. Buffer systems in the body. The ph of the blood is maintained between 7.35 and 7.45 by buffers. Important Buffer In Human Blood.
From slideplayer.com
Biological Buffers. ppt download Important Buffer In Human Blood The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the. Buffer systems in the body. In this system, gaseous metabolic waste carbon dioxide reacts. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. 3) are much greater than. In this. Important Buffer In Human Blood.
From ar.inspiredpencil.com
Renal Buffer System Important Buffer In Human Blood The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. If blood ph goes above or below that range, it can destroy cells. This buffer works well because concentrations of the buffer components hco. 3) are much greater than. The buffer systems in the human body are extremely efficient, and different systems work at. Important Buffer In Human Blood.
From fblt.cz
7. AcidBase Balance • Functions of Cells and Human Body Important Buffer In Human Blood 3) are much greater than. The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the. Buffer systems in the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. It. Important Buffer In Human Blood.
From ppt-online.org
Disorders of metabolism. (Subject 9) презентация онлайн Important Buffer In Human Blood The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. It takes only seconds for the chemical. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Buffer systems in the body. In this system, gaseous metabolic waste. Important Buffer In Human Blood.
From www.slideshare.net
02 hydrolysis. buffers__colloids Important Buffer In Human Blood Buffer systems in the body. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. If blood ph goes above or below that range, it can destroy cells. The buffer systems in the human body are extremely efficient, and different systems work at different. Important Buffer In Human Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Important Buffer In Human Blood It takes only seconds for the. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. 3) are much greater than. This buffer works well because concentrations of the buffer components hco. The buffer systems in the human body are extremely efficient, and different. Important Buffer In Human Blood.
From www.slideserve.com
PPT Buffers and Salts PowerPoint Presentation, free download ID5405242 Important Buffer In Human Blood It takes only seconds for the chemical. This buffer works well because concentrations of the buffer components hco. Buffer systems in the body. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer. Important Buffer In Human Blood.
From poorvi77.blogspot.com
Poorveez ParadyzMedimuseion major buffer systems in the body Important Buffer In Human Blood 3) are much greater than. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. This buffer works well because concentrations of the buffer components hco. It takes only seconds for the. If blood ph. Important Buffer In Human Blood.
From www.numerade.com
SOLVED Q The buffer systems that are the most important in the Important Buffer In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. 3) are much greater than. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. The ph of. Important Buffer In Human Blood.
From www.chegg.com
Solved Human blood contains several buffering systems, the Important Buffer In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. In this system, gaseous metabolic waste carbon dioxide reacts. If blood ph goes above or below that range, it can destroy cells. The buffer systems in the human body are extremely efficient, and different systems work at different rates. 3) are much greater than. The ph of the blood is maintained. Important Buffer In Human Blood.
From www.slideserve.com
PPT Biological buffering of blood PowerPoint Presentation, free Important Buffer In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. If blood ph goes above or below that range, it can destroy cells. 3) are much greater than. It takes only seconds for the chemical. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffer systems in the body. The buffer systems in. Important Buffer In Human Blood.
From www.slideserve.com
PPT Buffers and Titrations PowerPoint Presentation, free download Important Buffer In Human Blood Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems in the human body are extremely efficient, and different systems work at different rates. 3) are much greater than. The ph of. Important Buffer In Human Blood.
From www.slideserve.com
PPT General, Organic, and Biochemistry, 7e PowerPoint Presentation Important Buffer In Human Blood This buffer works well because concentrations of the buffer components hco. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. 3) are much greater than. Buffer. Important Buffer In Human Blood.
From www.slideserve.com
PPT Buffer Systems of the Body PowerPoint Presentation, free download Important Buffer In Human Blood 3) are much greater than. In this system, gaseous metabolic waste carbon dioxide reacts. This buffer works well because concentrations of the buffer components hco. Buffer systems in the body. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. In this system, gaseous. Important Buffer In Human Blood.
From www.bartleby.com
Answered Normally, the pH of the human body is… bartleby Important Buffer In Human Blood If blood ph goes above or below that range, it can destroy cells. It takes only seconds for the chemical. The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. The buffer systems in the human body are extremely efficient, and different systems work. Important Buffer In Human Blood.
From www.pinterest.com.au
Pin on Paramedici Important Buffer In Human Blood The buffer systems in the human body are extremely efficient, and different systems work at different rates. Buffer systems in the body. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order. Important Buffer In Human Blood.
From www.slideserve.com
PPT Physiological system of blood. Functional importance of blood Important Buffer In Human Blood It takes only seconds for the. The buffer systems in the human body are extremely efficient, and different systems work at different rates. In this system, gaseous metabolic waste carbon dioxide reacts. Buffer systems in the body. The buffer systems in the human body are extremely efficient, and different systems work at different rates. Human blood contains a buffer of. Important Buffer In Human Blood.
From www.slideshare.net
Buffer in the blood Important Buffer In Human Blood If blood ph goes above or below that range, it can destroy cells. 3) are much greater than. The ph of the blood is maintained between 7.35 and 7.45 by buffers in the body. In this system, gaseous metabolic waste carbon dioxide reacts. It takes only seconds for the chemical. It takes only seconds for the. The buffer systems in. Important Buffer In Human Blood.
From fblt.cz
7. AcidBase Balance • Functions of Cells and Human Body Important Buffer In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. 3) are much greater than. In this system, gaseous metabolic waste carbon dioxide reacts. Human blood contains a buffer of carbonic acid (h2co3 h 2 co 3) and bicarbonate anion (hco−3 hco 3 −) in order to maintain blood ph. If blood ph goes above or below that range, it can. Important Buffer In Human Blood.
From www.slideserve.com
PPT Buffers in Blood. Acidosis and Alkalosis. PowerPoint Presentation Important Buffer In Human Blood In this system, gaseous metabolic waste carbon dioxide reacts. If blood ph goes above or below that range, it can destroy cells. The buffer systems in the human body are extremely efficient, and different systems work at different rates. It takes only seconds for the chemical. The ph of the blood is maintained between 7.35 and 7.45 by buffers in. Important Buffer In Human Blood.