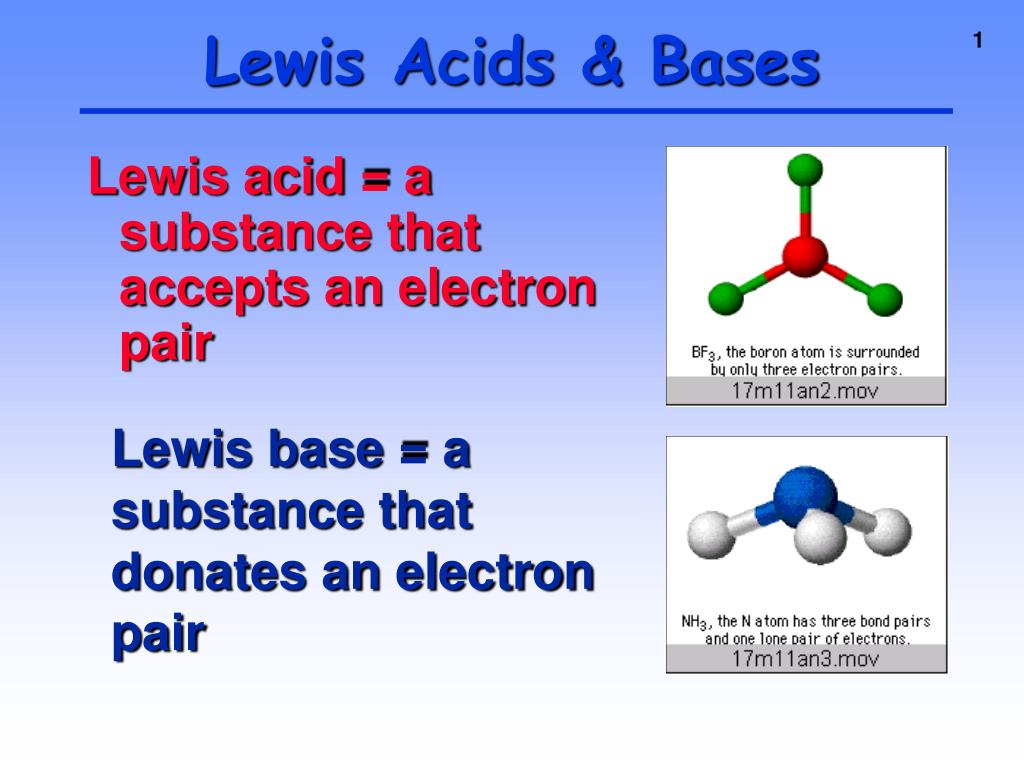
Difference Between Base And Lewis Base . Lewis bases are nucleophilic meaning that they “attack” a positive. Lewis bases donate an electron pair. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons.
from www.slideserve.com
In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases donate an electron pair. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor.
PPT Lewis Acids & Bases PowerPoint Presentation, free download ID
Difference Between Base And Lewis Base In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases donate an electron pair. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions.
From www.slideserve.com
PPT Chapter 14 PowerPoint Presentation, free download ID5372820 Difference Between Base And Lewis Base a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases donate an electron pair. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases are nucleophilic meaning that. Difference Between Base And Lewis Base.
From pediaa.com
Difference Between Acid and Base Difference Between Base And Lewis Base a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis base is defined as any species that. Difference Between Base And Lewis Base.
From www.chemistrylearner.com
Nucleophile Definition, Examples, and Strength Difference Between Base And Lewis Base Lewis bases donate an electron pair. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base. Difference Between Base And Lewis Base.
From ask.learncbse.in
Identify the Lewis acid and Lewis base in each of the following Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases donate an electron pair. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases. Difference Between Base And Lewis Base.
From mavink.com
Lewis Theory Of Acids And Bases Difference Between Base And Lewis Base a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases donate an electron pair. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. In this post, we will go. Difference Between Base And Lewis Base.
From www.pinterest.com
Lewis Acids and Bases Lewis acids and bases, Organic reactions, Chemistry Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. In this. Difference Between Base And Lewis Base.
From askanydifference.com
Lewis Acid vs Base Difference and Comparison Difference Between Base And Lewis Base In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases are nucleophilic meaning that they “attack”. Difference Between Base And Lewis Base.
From leah4sci.com
Definitions of Arrhenius, BronstedLowry, and Lewis Acids and Bases in Difference Between Base And Lewis Base a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases donate an electron pair. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis base. Difference Between Base And Lewis Base.
From www.slideserve.com
PPT Calculating Percent Ionization PowerPoint Presentation ID604587 Difference Between Base And Lewis Base In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases donate an electron pair. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. a. Difference Between Base And Lewis Base.
From keithkruwmitchell.blogspot.com
Lewis Acid and Base KeithkruwMitchell Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases are nucleophilic meaning that they “attack” a positive. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other. Difference Between Base And Lewis Base.
From www.expii.com
Lewis Acid and Bases — Definition & Examples Expii Difference Between Base And Lewis Base Lewis bases donate an electron pair. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. In this post, we will go. Difference Between Base And Lewis Base.
From mydigitalkemistry.com
What is Lewis concept of Acid and Base in Simple Words Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases donate an electron pair. a. Difference Between Base And Lewis Base.
From www.coursehero.com
[Solved] Describe the difference between Bronsted acids and bases Difference Between Base And Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. Lewis bases donate an electron pair. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any. Difference Between Base And Lewis Base.
From differencebetweenz.com
Difference between Lewis Acid and Base Difference Betweenz Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases donate an electron pair. a. Difference Between Base And Lewis Base.
From www.youtube.com
Classify the Substances as Lewis Acids or Lewis Bases Identifying Difference Between Base And Lewis Base a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases are nucleophilic meaning that they “attack” a positive. In this. Difference Between Base And Lewis Base.
From fyowknctp.blob.core.windows.net
What Is Definition Of Base In Chemistry at Carol Clinton blog Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases donate an electron pair. Lewis bases are nucleophilic meaning that. Difference Between Base And Lewis Base.
From www.coursehero.com
[Solved] identify lewis base and acids. Identifying Lewis acids and Difference Between Base And Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases donate an electron pair. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any. Difference Between Base And Lewis Base.
From www.slideserve.com
PPT Lewis Acids & Bases PowerPoint Presentation, free download ID Difference Between Base And Lewis Base In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases are nucleophilic meaning that they “attack” a positive. Lewis bases donate an electron pair. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base. Difference Between Base And Lewis Base.
From www.youtube.com
How To Identify Lewis Acids and Lewis Bases YouTube Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases are nucleophilic meaning that they “attack”. Difference Between Base And Lewis Base.
From www.slideserve.com
PPT AcidBase Concepts PowerPoint Presentation, free download ID Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. In this post, we will go over the key aspects, reactions of. Difference Between Base And Lewis Base.
From facts.net
17 Astounding Facts About Lewis AcidBase Theory Difference Between Base And Lewis Base a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases are nucleophilic meaning that they “attack” a positive. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases donate an electron pair. a lewis base. Difference Between Base And Lewis Base.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Brønsted acid (Brønsted Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases are nucleophilic meaning that they “attack” a positive. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other. Difference Between Base And Lewis Base.
From www.organicchemistrytutor.com
AcidBase Chemistry — Organic Chemistry Tutor Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases. Difference Between Base And Lewis Base.
From www.chemistrysteps.com
Lewis Acids and Bases Chemistry Steps Difference Between Base And Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. Lewis bases donate an electron pair. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. a lewis acid is an electron pair acceptor, while a lewis base is. Difference Between Base And Lewis Base.
From courses.lumenlearning.com
Relative Strengths of Acids and Bases Chemistry Atoms First Difference Between Base And Lewis Base In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases donate an electron pair. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base. Difference Between Base And Lewis Base.
From www.youtube.com
18.1 Lewis theory vs BronstedLowry theory (HL) YouTube Difference Between Base And Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. Lewis bases donate an electron pair. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. In this post, we will go over the key aspects, reactions of lewis acids. Difference Between Base And Lewis Base.
From www.chem.ucla.edu
Illustrated Glossary of Organic Chemistry Lewis acid Difference Between Base And Lewis Base Lewis bases donate an electron pair. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept. Difference Between Base And Lewis Base.
From www.u-helmich.de
LewisBase Difference Between Base And Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. Lewis bases donate an electron pair. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any. Difference Between Base And Lewis Base.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Difference Between Base And Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases donate an electron pair. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base. Difference Between Base And Lewis Base.
From sciencenotes.org
Lewis Acid and Base Theory Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases are nucleophilic meaning that they “attack” a positive. Lewis bases. Difference Between Base And Lewis Base.
From www.meta-synthesis.com
Lewis Acid Base Reaction Chemistry Chemogenesis Difference Between Base And Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. In this. Difference Between Base And Lewis Base.
From www.youtube.com
Lewis acids and Lewis bases YouTube Difference Between Base And Lewis Base In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair. Difference Between Base And Lewis Base.
From www.masterorganicchemistry.com
Nucleophilicity vs. Basicity Master Organic Chemistry Difference Between Base And Lewis Base Lewis bases are nucleophilic meaning that they “attack” a positive. a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. In this. Difference Between Base And Lewis Base.
From www.slideserve.com
PPT Acid Definitions PowerPoint Presentation, free download ID6398687 Difference Between Base And Lewis Base a lewis base is defined as any species that can donate a pair of electrons, and a lewis acid is any species that can accept a pair of electrons. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. Lewis bases donate an electron pair. a. Difference Between Base And Lewis Base.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Difference Between Base And Lewis Base a lewis acid is an electron pair acceptor, while a lewis base is an electron pair donor. Lewis bases are nucleophilic meaning that they “attack” a positive. In this post, we will go over the key aspects, reactions of lewis acids and bases and relation to other chemical reactions. a lewis base is defined as any species that. Difference Between Base And Lewis Base.