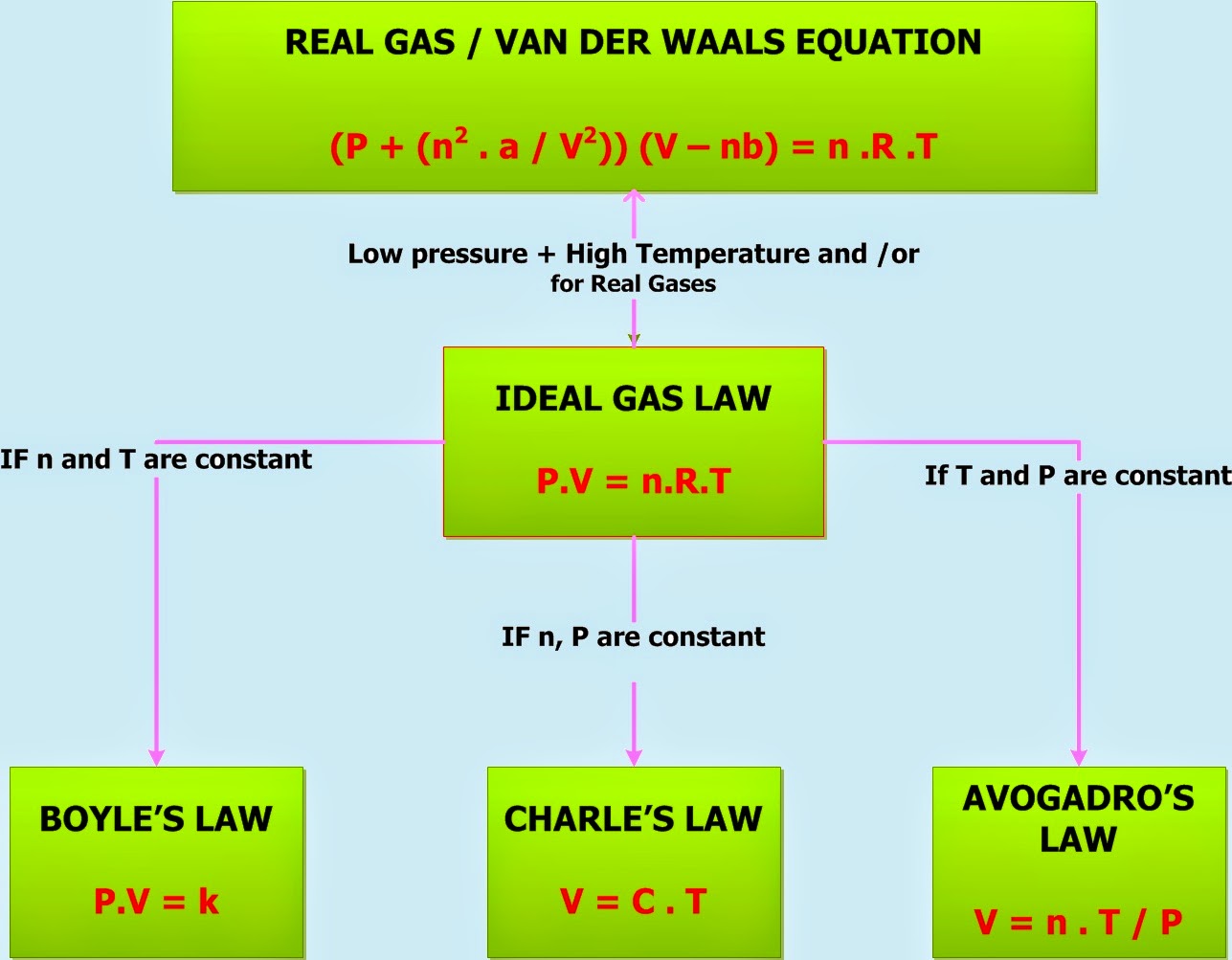
General Chemistry Gas Laws . the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the four variables used when discussing the physical behavior of any gas regardless of its identity are: It gives you a good intuition. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the gas laws are now explained by the microscopic behavior of gas molecules: It is a good approximation of the. you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. Charles' law, boyle's law and avogadro's law (all of which will later combine into. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. in such a case, all gases obey an equation of state known as the ideal gas law: the gas laws consist of three primary laws:
from chem-net.blogspot.com
the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the four variables used when discussing the physical behavior of any gas regardless of its identity are: It gives you a good intuition. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) Charles' law, boyle's law and avogadro's law (all of which will later combine into. you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. the gas laws consist of three primary laws: It is a good approximation of the. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the.
Gas Laws Ideal Gas Law Chemistry Net
General Chemistry Gas Laws the gas laws consist of three primary laws: in such a case, all gases obey an equation of state known as the ideal gas law: It gives you a good intuition. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the. the gas laws are now explained by the microscopic behavior of gas molecules: Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. Charles' law, boyle's law and avogadro's law (all of which will later combine into. the gas laws consist of three primary laws: (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) the four variables used when discussing the physical behavior of any gas regardless of its identity are: you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the.
From chemistry.coach
Acid Base Indicators Knowledge Base Chemistry Coach Page 3 General Chemistry Gas Laws It gives you a good intuition. a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) It is a good approximation of the. the ideal gas law, also called the general gas equation, is the equation of state. General Chemistry Gas Laws.
From www.chem.fsu.edu
Gas Laws General Chemistry Gas Laws the gas laws are now explained by the microscopic behavior of gas molecules: the four variables used when discussing the physical behavior of any gas regardless of its identity are: a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. in such a case, all gases obey an equation of state. General Chemistry Gas Laws.
From studyx.ai
8 Pada kondisi STP terdapat satu jenis gas StudyX General Chemistry Gas Laws Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. It is a good approximation of the. you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. the. General Chemistry Gas Laws.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube General Chemistry Gas Laws It is a good approximation of the. in such a case, all gases obey an equation of state known as the ideal gas law: the gas laws are now explained by the microscopic behavior of gas molecules: a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the ideal gas law,. General Chemistry Gas Laws.
From www.facebook.com
"Ask Indigo Brown on The Small Business Network?" The Hidden Forces General Chemistry Gas Laws in such a case, all gases obey an equation of state known as the ideal gas law: It gives you a good intuition. the four variables used when discussing the physical behavior of any gas regardless of its identity are: you can use the ideal gas law to make predictions about how gases will react when you. General Chemistry Gas Laws.
From msubbu.academy
Unit1 Basic Concepts CP and CV MSubbu Academy General Chemistry Gas Laws you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. the gas laws are now explained by the microscopic behavior of gas molecules: in such a case, all gases obey an equation of state known as the ideal gas law: It is a good approximation. General Chemistry Gas Laws.
From lessonlibwinters.z1.web.core.windows.net
Calculating Variables With Gas Laws General Chemistry Gas Laws in such a case, all gases obey an equation of state known as the ideal gas law: Charles' law, boyle's law and avogadro's law (all of which will later combine into. you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. Pv = nrt, where n. General Chemistry Gas Laws.
From ussanews.com
Argon (Ar) [Ne]3s23p6 Core Electrons and Valence Electrons Explained General Chemistry Gas Laws the gas laws consist of three primary laws: Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. the four variables used when discussing the physical behavior of any gas regardless of its identity are: the ideal gas law,. General Chemistry Gas Laws.
From chemistry101efhs.weebly.com
Gas Laws Chemistry 101 General Chemistry Gas Laws in such a case, all gases obey an equation of state known as the ideal gas law: the four variables used when discussing the physical behavior of any gas regardless of its identity are: Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules. General Chemistry Gas Laws.
From materialfullrobin.z13.web.core.windows.net
Gas Laws Equations Sheet General Chemistry Gas Laws the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. Charles' law, boyle's law. General Chemistry Gas Laws.
From sciencenotes.org
Ideal Gas Law Formula and Examples General Chemistry Gas Laws It is a good approximation of the. the gas laws are now explained by the microscopic behavior of gas molecules: Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. the four variables used when discussing the physical behavior of. General Chemistry Gas Laws.
From learningschooldirjenvd.z4.web.core.windows.net
Combined Gas Law Explanation General Chemistry Gas Laws It gives you a good intuition. It is a good approximation of the. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. in such a case, all gases obey an equation of state known as the ideal gas law: Charles'. General Chemistry Gas Laws.
From www.youtube.com
IDEAL GAS LAW PRACTICE PROBLEMS How to Solve Ideal Gas Law Problems General Chemistry Gas Laws the gas laws are now explained by the microscopic behavior of gas molecules: the four variables used when discussing the physical behavior of any gas regardless of its identity are: Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole.. General Chemistry Gas Laws.
From www.studypool.com
SOLUTION College general chemistry gas laws tutorial and answer key General Chemistry Gas Laws the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. in such a case, all gases obey an equation of state known as the ideal gas law: Charles' law, boyle's law and avogadro's law (all of which will later combine into. the gas laws consist of three. General Chemistry Gas Laws.
From mmerevise.co.uk
The Ideal Gas Equation MME General Chemistry Gas Laws the gas laws are now explained by the microscopic behavior of gas molecules: you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. It is a good approximation of the. the ideal gas law, also called the general gas equation, is the equation of state. General Chemistry Gas Laws.
From www.studocu.com
Gas Laws 5 General Chemistry Gas Laws 5 & 5. Ideal gas behaves General Chemistry Gas Laws Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. It is a good approximation of the. the gas laws are now explained by the microscopic behavior of gas molecules: the four variables used when discussing the physical behavior of. General Chemistry Gas Laws.
From www.studocu.com
Gas Laws Week 5&6 General Chemistry 1 Quarter 2 Module 1General General Chemistry Gas Laws It is a good approximation of the. It gives you a good intuition. you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. Charles' law, boyle's law and avogadro's law (all of which will later combine into. Pv = nrt, where n is the number of moles. General Chemistry Gas Laws.
From studyx.ai
Test Yosself Homework C Rozaimie Bin Wahab StudyX General Chemistry Gas Laws Charles' law, boyle's law and avogadro's law (all of which will later combine into. the gas laws consist of three primary laws: the four variables used when discussing the physical behavior of any gas regardless of its identity are: (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) a logical corollary. General Chemistry Gas Laws.
From cecfxdbt.blob.core.windows.net
Combined Gas Law Creator at Ernest Wilson blog General Chemistry Gas Laws Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. in such a case, all gases obey an equation of state known as the ideal gas law: the gas laws consist of three primary laws: the ideal gas law,. General Chemistry Gas Laws.
From mungfali.com
Gay Lussac's Law Worksheet General Chemistry Gas Laws the four variables used when discussing the physical behavior of any gas regardless of its identity are: you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. It gives you a good intuition. (the universal gas constant is defined as avogadro’s number na times the boltzmann. General Chemistry Gas Laws.
From studyx.ai
2 Pada suhu dan tekanan tertentu 2 liter gas StudyX General Chemistry Gas Laws It is a good approximation of the. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) the ideal gas law, also called the general gas. General Chemistry Gas Laws.
From www.britannica.com
Equation of state Definition, Ideal Gas, & Facts Britannica General Chemistry Gas Laws in such a case, all gases obey an equation of state known as the ideal gas law: the gas laws consist of three primary laws: (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) you can use the ideal gas law to make predictions about how gases will react when you. General Chemistry Gas Laws.
From dev.saylor.org
Saylor Academy All courses Saylor Academy General Chemistry Gas Laws a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. It is a good approximation of the. Charles' law, boyle's law and avogadro's law (all of which will later combine into. (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) in such a case, all gases obey. General Chemistry Gas Laws.
From www.facebook.com
AJAABALE IROYIN AJAABALE IROYIN w/ Samuel Gbesabi & Enitan Olusegun General Chemistry Gas Laws the four variables used when discussing the physical behavior of any gas regardless of its identity are: you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. in such a case, all gases obey an equation of state known as the ideal gas law: It. General Chemistry Gas Laws.
From mungfali.com
Gas Laws Formula Sheet General Chemistry Gas Laws Charles' law, boyle's law and avogadro's law (all of which will later combine into. in such a case, all gases obey an equation of state known as the ideal gas law: the four variables used when discussing the physical behavior of any gas regardless of its identity are: the ideal gas law, also called the general gas. General Chemistry Gas Laws.
From studylib.net
Gas Law Worksheet 2 General Chemistry Gas Laws Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. the gas laws are now explained by the microscopic behavior of gas molecules: Charles' law, boyle's law and avogadro's law (all of which will later combine into. a logical corollary. General Chemistry Gas Laws.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net General Chemistry Gas Laws It gives you a good intuition. Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. Charles' law, boyle's. General Chemistry Gas Laws.
From www.slideshare.net
Gas lawscheat sheet General Chemistry Gas Laws a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the gas laws are now explained by the microscopic behavior of gas molecules: It gives you a good intuition. Charles' law, boyle's law and avogadro's law (all of which will later combine into. the four variables used when discussing the physical behavior. General Chemistry Gas Laws.
From proper-cooking.info
Chemistry Conversion Chart Cheat Sheet General Chemistry Gas Laws you can use the ideal gas law to make predictions about how gases will react when you change pressure, volume or temperature. Charles' law, boyle's law and avogadro's law (all of which will later combine into. It is a good approximation of the. It gives you a good intuition. the gas laws consist of three primary laws: . General Chemistry Gas Laws.
From printablelibrarywills.z14.web.core.windows.net
Calculating Variables With Gas Laws General Chemistry Gas Laws in such a case, all gases obey an equation of state known as the ideal gas law: It is a good approximation of the. the gas laws consist of three primary laws: a logical corollary to avogadro's hypothesis (sometimes called avogadro’s law) describes the relationship between the. the gas laws are now explained by the microscopic. General Chemistry Gas Laws.
From www.studocu.com
Discussion 4 page 2 1. M moles 18 moles 7 moles 2. J J Thom Atoms are General Chemistry Gas Laws Charles' law, boyle's law and avogadro's law (all of which will later combine into. in such a case, all gases obey an equation of state known as the ideal gas law: It is a good approximation of the. the gas laws are now explained by the microscopic behavior of gas molecules: (the universal gas constant is defined as. General Chemistry Gas Laws.
From studyx.ai
Trabaja Elabora ACTIVIDAD 3 individual TI A StudyX General Chemistry Gas Laws the four variables used when discussing the physical behavior of any gas regardless of its identity are: (the universal gas constant is defined as avogadro’s number na times the boltzmann constant k.) It is a good approximation of the. the gas laws consist of three primary laws: It gives you a good intuition. Pv = nrt, where n. General Chemistry Gas Laws.
From studyx.ai
Amonia dapat dibuat melalui reaksi N2 g H2 g StudyX General Chemistry Gas Laws the gas laws are now explained by the microscopic behavior of gas molecules: Pv = nrt, where n is the number of moles of the gas and r is the universal (or perfect) gas constant, 8.31446261815324 joules per kelvin per mole. Charles' law, boyle's law and avogadro's law (all of which will later combine into. the ideal gas. General Chemistry Gas Laws.
From oertx.highered.texas.gov
General Chemistry for Science Majors, Unit 3, Gas Laws OERTX General Chemistry Gas Laws in such a case, all gases obey an equation of state known as the ideal gas law: the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. the four variables used when discussing the physical behavior of any gas regardless of its identity are: a logical. General Chemistry Gas Laws.
From studiousguy.com
The Gas Laws Definition, Formula & Examples StudiousGuy General Chemistry Gas Laws the gas laws consist of three primary laws: Charles' law, boyle's law and avogadro's law (all of which will later combine into. the ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the. a logical corollary to avogadro's hypothesis (sometimes called. General Chemistry Gas Laws.