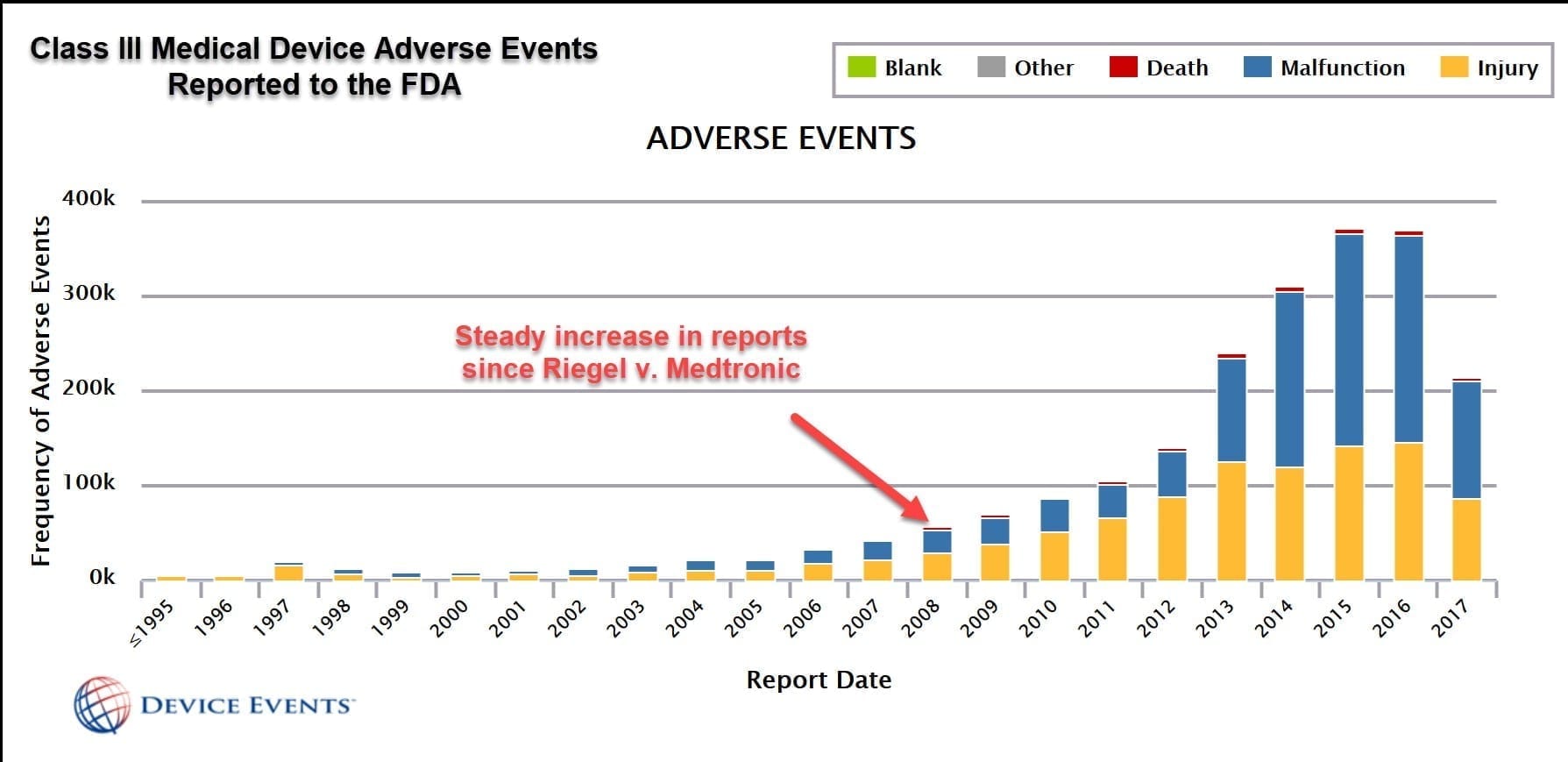
Medical Device Safety Act . “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. The eu commission introduced two new regulations for medical devices. the medical devices regulation (mdr) has been in force since 26 may 2021. Main features of the mdr. what is the purpose of the medical device regulations? how are medical devices and ivds regulated? (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. This webpage covers topics that are specific to. The new medical devices regulation (mdr). The medical devices area of our website includes safety updates for healthcare professionals and those who.
from www.legalreader.com
Main features of the mdr. the medical devices regulation (mdr) has been in force since 26 may 2021. what is the purpose of the medical device regulations? how are medical devices and ivds regulated? (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. The new medical devices regulation (mdr). the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. This webpage covers topics that are specific to. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. The medical devices area of our website includes safety updates for healthcare professionals and those who.
Medical Device Safety Act Aims to Restore Rights to Patients Harmed
Medical Device Safety Act The new medical devices regulation (mdr). The eu commission introduced two new regulations for medical devices. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. The medical devices area of our website includes safety updates for healthcare professionals and those who. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. what is the purpose of the medical device regulations? the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. This webpage covers topics that are specific to. how are medical devices and ivds regulated? Main features of the mdr. the medical devices regulation (mdr) has been in force since 26 may 2021. The new medical devices regulation (mdr).
From www.edwardjamesletko.com
Assuring Medical Device Safety Edward James Letko Medical Device Safety Act the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons,. Medical Device Safety Act.
From healthcare-international.meti.go.jp
MEDICAL DEVICE ACT 2012 (ACT 737)(UPDATES ON MEDICAL DEVICE ACT Medical Device Safety Act Main features of the mdr. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. the medical devices regulation (mdr) has been in force since 26 may 2021. The new medical devices regulation (mdr). The eu commission introduced two new regulations for medical devices.. Medical Device Safety Act.
From betebt.com
Medical Device Regulation Importance and Examples in APAC (2022) Medical Device Safety Act Main features of the mdr. how are medical devices and ivds regulated? (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. The new medical devices regulation (mdr). The eu commission introduced two new regulations for medical devices. what is the purpose of. Medical Device Safety Act.
From www.legalreader.com
Medical Device Safety Act Aims to Restore Rights to Patients Harmed Medical Device Safety Act the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. the medical devices regulation (mdr) has been in force since 26 may 2021. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. (b) a device presents. Medical Device Safety Act.
From www.slideshare.net
Safe Medical Devices Act 1990 Medical Device Safety Act the medical devices regulation (mdr) has been in force since 26 may 2021. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. how are medical devices and ivds regulated? The new medical devices regulation (mdr). Main features of the mdr. what. Medical Device Safety Act.
From www.slideshare.net
Health and safety act Medical Device Safety Act the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. what is the purpose of the medical device regulations? This webpage covers topics that are specific to. The. Medical Device Safety Act.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Safety Act The medical devices area of our website includes safety updates for healthcare professionals and those who. The new medical devices regulation (mdr). the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to. Medical Device Safety Act.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Device Safety Act (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. Main features of the mdr. The medical devices area of our website includes safety updates for healthcare professionals and those who. The new medical devices regulation (mdr). “device” means— (a) a medical device, (b). Medical Device Safety Act.
From exyuxzjps.blob.core.windows.net
Medical Device Regulations Fda at Lois Ogrady blog Medical Device Safety Act the medical devices regulation (mdr) has been in force since 26 may 2021. how are medical devices and ivds regulated? “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. The medical devices area of our website includes safety updates for healthcare professionals. Medical Device Safety Act.
From www.bol.com
H.R. 1346, the Medical Device Safety Act of 2009, United States House Medical Device Safety Act This webpage covers topics that are specific to. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. how. Medical Device Safety Act.
From medtruth.com
How To Support The Medical Device Safety Act MedTruth Prescription Medical Device Safety Act the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. The new medical devices regulation (mdr). This webpage covers topics that are specific to. The medical devices area of our website includes safety updates for healthcare professionals and those who. the medical devices regulation (mdr) has been in force since 26 may 2021.. Medical Device Safety Act.
From www.change.org
Petition · Support The Medical Device Safety Act United States Medical Device Safety Act how are medical devices and ivds regulated? the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. The new medical devices regulation (mdr). This webpage covers topics that are specific to. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other. Medical Device Safety Act.
From medtruth.medium.com
Why the Medical Device Safety Act Matters by MedTruth Medium Medical Device Safety Act This webpage covers topics that are specific to. The new medical devices regulation (mdr). “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. what is the purpose of the medical device regulations? the medical devices regulation (mdr) has been in force since. Medical Device Safety Act.
From www.scribd.com
Introduction to Medical Device Act 2012 (Act 737) and Medical Device Medical Device Safety Act the medical devices regulation (mdr) has been in force since 26 may 2021. Main features of the mdr. The eu commission introduced two new regulations for medical devices. how are medical devices and ivds regulated? The new medical devices regulation (mdr). “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product. Medical Device Safety Act.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Medical Device Safety Act The medical devices area of our website includes safety updates for healthcare professionals and those who. the medical devices regulation (mdr) has been in force since 26 may 2021. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. what is the purpose. Medical Device Safety Act.
From www.homecarepulse.com
Safe Medical Devices Act Home Care Pulse Medical Device Safety Act the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. how are medical devices and ivds regulated? what is the purpose of the medical device regulations? the medical devices regulation (mdr) has been in force since 26 may 2021. “device” means— (a) a medical device, (b) an accessory for a. Medical Device Safety Act.
From www.healthybdaily.com
General Medical Device Safety Tips Healthy B Daily Medical Device Safety Act the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. The medical devices area of our website includes safety updates for healthcare professionals and those who. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. This webpage covers. Medical Device Safety Act.
From www.pdffiller.com
Fillable Online Why You Should Care About The Medical Device Safety Act Medical Device Safety Act what is the purpose of the medical device regulations? “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. the medical devices regulation (mdr) has been in force since 26 may 2021. the new regulations ultimately aim to provide a secure, consistent. Medical Device Safety Act.
From elexacademicbookstore.co.za
Occupational Health and Safety Act 85 of 1993 Poster Elex Academic Medical Device Safety Act how are medical devices and ivds regulated? the medical devices regulation (mdr) has been in force since 26 may 2021. what is the purpose of the medical device regulations? the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. (b) a device presents an unacceptable risk to the health or. Medical Device Safety Act.
From www.makrosafe.co.za
5 Things you must know about the Health and Safety Act Medical Device Safety Act This webpage covers topics that are specific to. what is the purpose of the medical device regulations? how are medical devices and ivds regulated? the medical devices regulation (mdr) has been in force since 26 may 2021. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex. Medical Device Safety Act.
From mdcpublishers.com
Health Medical Device Act 2012 Medical Device Safety Act Main features of the mdr. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. how are medical devices and ivds regulated? (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other. Medical Device Safety Act.
From www.slideserve.com
PPT Overview of FDA How Regulation Came to Be PowerPoint Medical Device Safety Act (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. Main features of the mdr. This webpage covers topics that are specific to. The medical devices area of our website includes safety updates for healthcare professionals and those who. the medical devices regulation (mdr). Medical Device Safety Act.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Safety Act what is the purpose of the medical device regulations? the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. Main features of the mdr. how are medical devices and ivds regulated? (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to. Medical Device Safety Act.
From medicaldeviceproblems.com
Patient Advocacy Archives Medical Device Problems Medical Device Safety Act how are medical devices and ivds regulated? the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. This webpage covers topics that are specific to. what is the purpose of the medical device regulations? The new medical devices regulation (mdr). the medical devices regulation (mdr) has been in force since 26. Medical Device Safety Act.
From www.asianhhm.com
Regulation And Monitoring Of Medical Devices In Global Realms Of World Medical Device Safety Act how are medical devices and ivds regulated? Main features of the mdr. what is the purpose of the medical device regulations? The eu commission introduced two new regulations for medical devices. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. The medical. Medical Device Safety Act.
From rbccorp.com
Some of the Most Critical Medical Device Standards Medical Device Safety Act The new medical devices regulation (mdr). the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. how are medical devices and ivds regulated? The eu commission introduced two new regulations for medical devices. the medical devices regulation (mdr) has been in force since 26 may 2021. The medical devices area of our. Medical Device Safety Act.
From shotnelo.weebly.com
Medical device risk assessment template shotnelo Medical Device Safety Act how are medical devices and ivds regulated? the medical devices regulation (mdr) has been in force since 26 may 2021. the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. This webpage covers topics that are specific to. The eu commission introduced two new regulations for medical devices. The medical devices area. Medical Device Safety Act.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Safety Act This webpage covers topics that are specific to. Main features of the mdr. the medical devices regulation (mdr) has been in force since 26 may 2021. The eu commission introduced two new regulations for medical devices. The medical devices area of our website includes safety updates for healthcare professionals and those who. (b) a device presents an unacceptable. Medical Device Safety Act.
From www.slideserve.com
PPT Tort Reform in Reverse? PowerPoint Presentation ID4881215 Medical Device Safety Act The eu commission introduced two new regulations for medical devices. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. The medical devices area of our website includes safety updates for healthcare professionals and those who. how are medical devices and ivds regulated? . Medical Device Safety Act.
From www.youtube.com
Please support the Medical Device Safety Act YouTube Medical Device Safety Act The eu commission introduced two new regulations for medical devices. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. the new regulations ultimately aim to provide a secure, consistent regulatory framework for all medical. The new medical devices regulation (mdr). The medical devices. Medical Device Safety Act.
From www.goldbamboo.com
Drug and Medical Device Safety as related to Safety Pictures Medical Device Safety Act This webpage covers topics that are specific to. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. what. Medical Device Safety Act.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Device Safety Act The new medical devices regulation (mdr). The medical devices area of our website includes safety updates for healthcare professionals and those who. Main features of the mdr. The eu commission introduced two new regulations for medical devices. how are medical devices and ivds regulated? the medical devices regulation (mdr) has been in force since 26 may 2021. . Medical Device Safety Act.
From www.apcerls.com
Safety & Regulatory requirements for Medical Devices APCER Life Sciences Medical Device Safety Act Main features of the mdr. The eu commission introduced two new regulations for medical devices. This webpage covers topics that are specific to. “device” means— (a) a medical device, (b) an accessory for a medical device, (c) a product listed in annex xvi to the medical devices. how are medical devices and ivds regulated? the medical devices. Medical Device Safety Act.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Device Safety Act This webpage covers topics that are specific to. how are medical devices and ivds regulated? the medical devices regulation (mdr) has been in force since 26 may 2021. (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the. The new medical devices regulation. Medical Device Safety Act.
From www.palomar.edu
Video Cause supporters rally in Washington, DC The Telescope Medical Device Safety Act The medical devices area of our website includes safety updates for healthcare professionals and those who. how are medical devices and ivds regulated? Main features of the mdr. The new medical devices regulation (mdr). (b) a device presents an unacceptable risk to the health or safety of patients, users or other persons, or to other aspects of the.. Medical Device Safety Act.