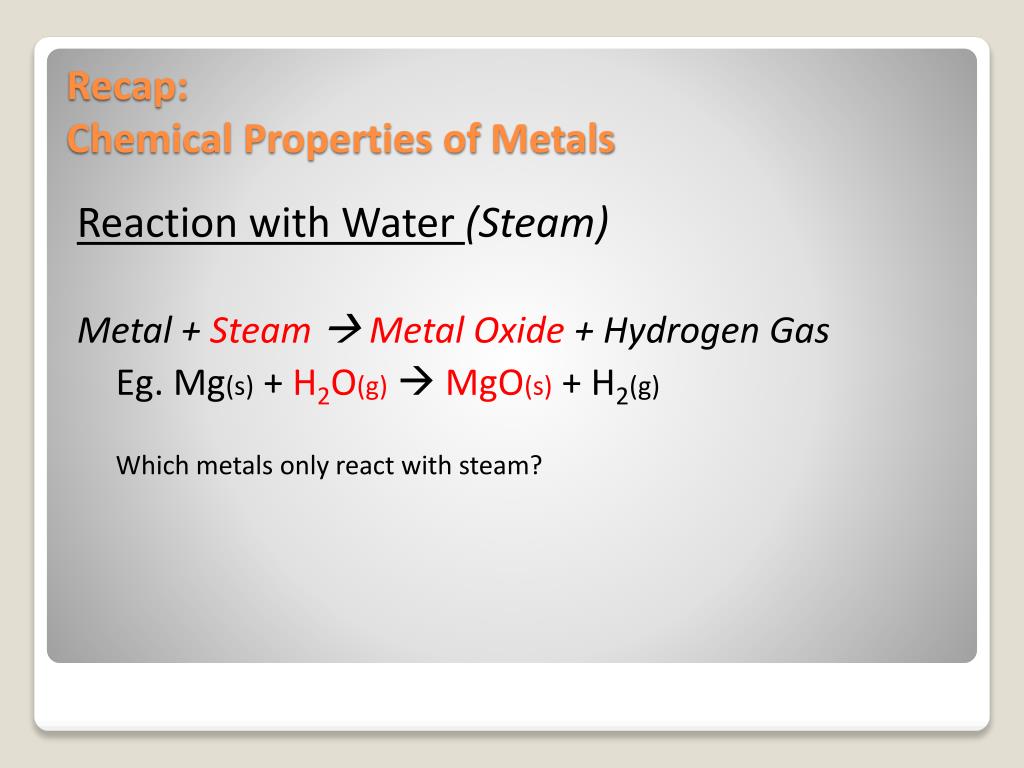
Metal Reaction With Water Examples . The general equation for these. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. Potassium reacts quickly with water. when metals react with water, metal hydroxides and hydrogen gas are formed. reactions of metals with water. metals from calcium upwards. These react vigorously (and even more vigorously the higher up the series you go) with cold. When a metal reacts with water, a metal hydroxide and hydrogen are formed. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. when a metal reacts with water, a metal hydroxide and hydrogen are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. The nonmetal oxides react with water to form oxoacids.
from www.slideserve.com
when metals react with water, metal hydroxides and hydrogen gas are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. When a metal reacts with water, a metal hydroxide and hydrogen are formed. The general equation for these. These react vigorously (and even more vigorously the higher up the series you go) with cold. The nonmetal oxides react with water to form oxoacids. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. Potassium reacts quickly with water. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. metals from calcium upwards.
PPT Reactivity Series of Metals PowerPoint Presentation ID468914
Metal Reaction With Water Examples When a metal reacts with water, a metal hydroxide and hydrogen are formed. reactions of metals with water. metals from calcium upwards. Potassium reacts quickly with water. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. when metals react with water, metal hydroxides and hydrogen gas are formed. The nonmetal oxides react with water to form oxoacids. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. These react vigorously (and even more vigorously the higher up the series you go) with cold. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. The general equation for these. when a metal reacts with water, a metal hydroxide and hydrogen are formed. When a metal reacts with water, a metal hydroxide and hydrogen are formed.
From ppt-online.org
Metals презентация онлайн Metal Reaction With Water Examples These react vigorously (and even more vigorously the higher up the series you go) with cold. The nonmetal oxides react with water to form oxoacids. Potassium reacts quickly with water. When a metal reacts with water, a metal hydroxide and hydrogen are formed. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas.. Metal Reaction With Water Examples.
From www.goodscience.com.au
Metal Reactions Good Science Metal Reaction With Water Examples metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. These react vigorously (and even more vigorously the higher up the series you go) with cold. reactions of metals with water. as mentioned. Metal Reaction With Water Examples.
From m20131000606.blogspot.com
Chemistry Reactivity of Alkali Metal with Chlorine, Oxygen and Water Metal Reaction With Water Examples When a metal reacts with water, a metal hydroxide and hydrogen are formed. The general equation for these. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. when metals react with water, metal hydroxides and hydrogen gas are formed. These react vigorously (and even more vigorously the higher up the series. Metal Reaction With Water Examples.
From www.youtube.com
Reaction of metals with water Class 10 Chemistry ICSE Board Metal Reaction With Water Examples reactions of metals with water. when metals react with water, metal hydroxides and hydrogen gas are formed. metals from calcium upwards. The nonmetal oxides react with water to form oxoacids. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. when a metal reacts with water, a metal hydroxide. Metal Reaction With Water Examples.
From www.teachoo.com
Reaction of Metals and NonMetals with Acids Teachoo Concepts Metal Reaction With Water Examples reactions of metals with water. when metals react with water, metal hydroxides and hydrogen gas are formed. These react vigorously (and even more vigorously the higher up the series you go) with cold. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Potassium reacts quickly with water. The nonmetal. Metal Reaction With Water Examples.
From www.youtube.com
Alkali metalwater reaction in slow motion YouTube Metal Reaction With Water Examples as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. when metals react with water, metal hydroxides and hydrogen gas are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. metals from calcium upwards. When a metal reacts with water,. Metal Reaction With Water Examples.
From www.chemistrystudent.com
Group 2 Metal Reactions (ALevel) ChemistryStudent Metal Reaction With Water Examples when a metal reacts with water, a metal hydroxide and hydrogen are formed. metals from calcium upwards. These react vigorously (and even more vigorously the higher up the series you go) with cold. The nonmetal oxides react with water to form oxoacids. When a metal reacts with water, a metal hydroxide and hydrogen are formed. reactions of. Metal Reaction With Water Examples.
From www.youtube.com
How acids react with metallic oxides CBSE Class 10 Chemistry Notes Metal Reaction With Water Examples The general equation for these. Potassium reacts quickly with water. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. when metals react with water, metal hydroxides and hydrogen gas are formed. when a metal reacts with water, a metal hydroxide and hydrogen are formed. These react vigorously (and even. Metal Reaction With Water Examples.
From www.bbc.co.uk
BBC Two Bitesize Chemistry, Alkali metals and their reactions to air Metal Reaction With Water Examples reactions of metals with water. When a metal reacts with water, a metal hydroxide and hydrogen are formed. The nonmetal oxides react with water to form oxoacids. when a metal reacts with water, a metal hydroxide and hydrogen are formed. metals from calcium upwards. The general equation for these. metals generally react more readily with dilute. Metal Reaction With Water Examples.
From abhyasonline.in
Concept Explanation Metal Reaction With Water Examples when a metal reacts with water, a metal hydroxide and hydrogen are formed. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. When a metal reacts with water, a metal hydroxide and hydrogen are formed. The general equation for these. all of group 1 elements—lithium, sodium, potassium, rubidium and. Metal Reaction With Water Examples.
From www.youtube.com
Sodium metal reaction with water YouTube Metal Reaction With Water Examples as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. reactions of metals with water. Potassium reacts quickly with water. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. These react vigorously (and even more vigorously the higher up the series you go). Metal Reaction With Water Examples.
From general.chemistrysteps.com
Displacement Reactions Chemistry Steps Metal Reaction With Water Examples Potassium reacts quickly with water. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. The nonmetal oxides react with water to form oxoacids. when a metal reacts with water, a metal hydroxide and hydrogen are formed. metals from calcium upwards. These react vigorously (and even more vigorously the higher up. Metal Reaction With Water Examples.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 2.1.1 Group 1 (Alkali Metals)翰林国际教育 Metal Reaction With Water Examples When a metal reacts with water, a metal hydroxide and hydrogen are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. The nonmetal oxides react with water to form oxoacids. reactions of metals with water. Potassium reacts quickly with water. These react vigorously (and even more vigorously the higher. Metal Reaction With Water Examples.
From fyoqjyjrc.blob.core.windows.net
Metal + Water = Metal Oxide + Hydrogen Example at Edward Ray blog Metal Reaction With Water Examples when metals react with water, metal hydroxides and hydrogen gas are formed. When a metal reacts with water, a metal hydroxide and hydrogen are formed. reactions of metals with water. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. These react vigorously (and even more vigorously the higher up. Metal Reaction With Water Examples.
From readthescience.blogspot.com
READ THE SCIENCE 10.1 The reaction of metals with oxygen Metal Reaction With Water Examples These react vigorously (and even more vigorously the higher up the series you go) with cold. The nonmetal oxides react with water to form oxoacids. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. Potassium reacts quickly with water. metals from calcium upwards. all of group 1 elements—lithium, sodium, potassium,. Metal Reaction With Water Examples.
From www.youtube.com
CHEMISTRY 101 Combustion reactions, reactions with alkali metals, and Metal Reaction With Water Examples reactions of metals with water. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. The general equation for these. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. The nonmetal oxides react with water to form oxoacids. when a metal reacts. Metal Reaction With Water Examples.
From www.slideserve.com
PPT Reactivity Series of Metals PowerPoint Presentation ID468914 Metal Reaction With Water Examples as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. metals from calcium upwards. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. when a metal reacts with water, a metal hydroxide and hydrogen are formed. when metals react with. Metal Reaction With Water Examples.
From ar.inspiredpencil.com
Metal And Water Reaction Metal Reaction With Water Examples metals from calcium upwards. when metals react with water, metal hydroxides and hydrogen gas are formed. The general equation for these. when a metal reacts with water, a metal hydroxide and hydrogen are formed. Potassium reacts quickly with water. These react vigorously (and even more vigorously the higher up the series you go) with cold. The nonmetal. Metal Reaction With Water Examples.
From www.youtube.com
Reactions Of Metals With Water Reactions Chemistry FuseSchool Metal Reaction With Water Examples as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. The nonmetal oxides react with water to form oxoacids. When a metal reacts with water, a metal hydroxide and hydrogen are formed. when. Metal Reaction With Water Examples.
From www.onlinemathlearning.com
Reaction of Metals (examples, answers, activities, experiment, videos) Metal Reaction With Water Examples when a metal reacts with water, a metal hydroxide and hydrogen are formed. These react vigorously (and even more vigorously the higher up the series you go) with cold. When a metal reacts with water, a metal hydroxide and hydrogen are formed. when metals react with water, metal hydroxides and hydrogen gas are formed. The nonmetal oxides react. Metal Reaction With Water Examples.
From www.teachoo.com
Reaction of Metals and Nonmetals with Water with examples Teachoo Metal Reaction With Water Examples when a metal reacts with water, a metal hydroxide and hydrogen are formed. The general equation for these. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. When a metal reacts with water, a metal hydroxide and hydrogen are formed. reactions of metals with water. These react vigorously (and. Metal Reaction With Water Examples.
From byjus.com
2.Why metals don't react with water? Metal Reaction With Water Examples when metals react with water, metal hydroxides and hydrogen gas are formed. reactions of metals with water. When a metal reacts with water, a metal hydroxide and hydrogen are formed. The general equation for these. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. when a metal reacts. Metal Reaction With Water Examples.
From www.meritnation.com
Reaction of metals with water explain with examples the whole concept Metal Reaction With Water Examples Potassium reacts quickly with water. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. metals from calcium upwards. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. The general equation for these. metals generally react more readily with dilute acids. Metal Reaction With Water Examples.
From www.slideserve.com
PPT Reacting metals with oxygen PowerPoint Presentation, free Metal Reaction With Water Examples when metals react with water, metal hydroxides and hydrogen gas are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. when a metal reacts with water, a metal hydroxide and hydrogen are formed. The general equation for these. Potassium reacts quickly with water. metals from calcium upwards.. Metal Reaction With Water Examples.
From www.science-revision.co.uk
Reactions of alkali metals Metal Reaction With Water Examples The nonmetal oxides react with water to form oxoacids. when metals react with water, metal hydroxides and hydrogen gas are formed. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. The general equation for these. metals from calcium upwards. reactions of metals with water. as mentioned earlier, many. Metal Reaction With Water Examples.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:2.4.1 Metals Reacting with Metal Reaction With Water Examples as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. Potassium reacts quickly with water. When a metal reacts with water, a metal hydroxide and hydrogen are formed. The nonmetal oxides react with water to form oxoacids. when metals react with water, metal hydroxides and hydrogen gas are formed. metals. Metal Reaction With Water Examples.
From www.doubtnut.com
Apparatus Beakers. Chemicals Samples of various metals , water. Metal Reaction With Water Examples all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Potassium reacts quickly with water. When a metal reacts with water, a metal hydroxide and hydrogen are formed. when a metal reacts with water, a metal hydroxide and hydrogen are formed. reactions of metals with water. metals from calcium. Metal Reaction With Water Examples.
From www.teachoo.com
Reaction of Metals and Nonmetals With Base Teachoo Concepts Metal Reaction With Water Examples metals from calcium upwards. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. when a metal reacts with water, a metal hydroxide and hydrogen are formed. when metals react with water, metal hydroxides and hydrogen gas are formed. These react vigorously (and even more vigorously the higher up the. Metal Reaction With Water Examples.
From www.slideserve.com
PPT METAL REACTIONS PowerPoint Presentation, free download ID2670563 Metal Reaction With Water Examples when metals react with water, metal hydroxides and hydrogen gas are formed. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. The general equation for these. when a metal reacts with water, a metal hydroxide and hydrogen are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and. Metal Reaction With Water Examples.
From www.bbc.co.uk
What is an acid and metal reaction? BBC Bitesize Metal Reaction With Water Examples The general equation for these. When a metal reacts with water, a metal hydroxide and hydrogen are formed. reactions of metals with water. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. The nonmetal oxides react with water to form oxoacids. when a metal reacts with water, a metal hydroxide. Metal Reaction With Water Examples.
From www.pinterest.com
Alkali metals react with water to produce hydroxides. Alkali metal Metal Reaction With Water Examples These react vigorously (and even more vigorously the higher up the series you go) with cold. The nonmetal oxides react with water to form oxoacids. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas.. Metal Reaction With Water Examples.
From www.thesciencehive.co.uk
The Periodic Table (AQA) — the science hive Metal Reaction With Water Examples metals generally react more readily with dilute acids than with water, producing salt and hydrogen gas. Potassium reacts quickly with water. metals from calcium upwards. when a metal reacts with water, a metal hydroxide and hydrogen are formed. These react vigorously (and even more vigorously the higher up the series you go) with cold. When a metal. Metal Reaction With Water Examples.
From www.teachoo.com
Reaction of Metals and Nonmetals with Oxygen Concepts Metal Reaction With Water Examples These react vigorously (and even more vigorously the higher up the series you go) with cold. metals from calcium upwards. Potassium reacts quickly with water. The general equation for these. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium. Metal Reaction With Water Examples.
From www.chemistryscl.com
Alkaline Earth Metals Reactions, Uses, Chemical and Physical Properties Metal Reaction With Water Examples all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously or even explosively with cold. Potassium reacts quickly with water. as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. reactions of metals with water. when metals react with water, metal hydroxides and hydrogen gas are formed.. Metal Reaction With Water Examples.
From www.teachoo.com
How do Acids and Bases react with Metals? [with Examples] Teachoo Metal Reaction With Water Examples as mentioned earlier, many group 1 and group 2 oxides react with water to form metal hydroxides. These react vigorously (and even more vigorously the higher up the series you go) with cold. When a metal reacts with water, a metal hydroxide and hydrogen are formed. all of group 1 elements—lithium, sodium, potassium, rubidium and cesium react vigorously. Metal Reaction With Water Examples.