Can You Compress Air Into A Liquid . Think of it as a large room of bouncing balls. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. This increased density makes the molecules move faster, increasing. Can you compress air into a liquid? Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. If you compress liquid, it doesn't have anywhere to go. Bulk modulus is a measure of a substance's resistance to uniform compression. This involves compressing air using a. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. If all the balls are bouncing really quickly, you would need additional space. It only seems like it because you can see gases be compressed, but you can't see it in liquids. When you compress air, you force its molecules into a smaller space.
from www.slideserve.com
If all the balls are bouncing really quickly, you would need additional space. If you compress liquid, it doesn't have anywhere to go. When you compress air, you force its molecules into a smaller space. This involves compressing air using a. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. Bulk modulus is a measure of a substance's resistance to uniform compression. Can you compress air into a liquid? Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. This increased density makes the molecules move faster, increasing. Think of it as a large room of bouncing balls.
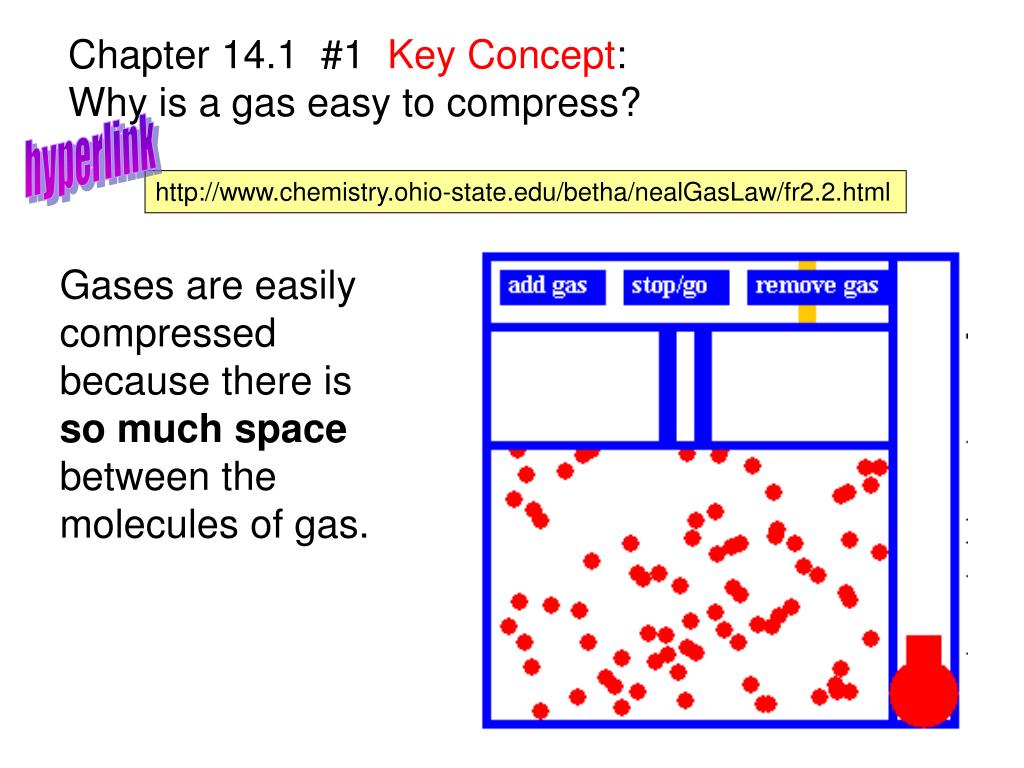
PPT Chapter 14.1 1 Key Concept Why is a gas easy to compress
Can You Compress Air Into A Liquid If you compress liquid, it doesn't have anywhere to go. Bulk modulus is a measure of a substance's resistance to uniform compression. It only seems like it because you can see gases be compressed, but you can't see it in liquids. When you compress air, you force its molecules into a smaller space. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. If all the balls are bouncing really quickly, you would need additional space. Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. If you compress liquid, it doesn't have anywhere to go. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. This involves compressing air using a. Can you compress air into a liquid? Think of it as a large room of bouncing balls. This increased density makes the molecules move faster, increasing.
From www.britannica.com
Compressed air Energy Efficiency, Industrial Uses & Safety Britannica Can You Compress Air Into A Liquid Think of it as a large room of bouncing balls. If you compress liquid, it doesn't have anywhere to go. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. It only seems like it because you can see gases be. Can You Compress Air Into A Liquid.
From www.youtube.com
Unit 1 Thermodynamics Compressed liquid approximations YouTube Can You Compress Air Into A Liquid If all the balls are bouncing really quickly, you would need additional space. This increased density makes the molecules move faster, increasing. Can you compress air into a liquid? Bulk modulus is a measure of a substance's resistance to uniform compression. Think of it as a large room of bouncing balls. Air is a mixture of nitrogen, oxygen and other. Can You Compress Air Into A Liquid.
From www.youtube.com
Adiabatic compression and expansion of a gas HD YouTube Can You Compress Air Into A Liquid This involves compressing air using a. Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. Bulk modulus is a measure of a substance's resistance to uniform compression. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to. Can You Compress Air Into A Liquid.
From www.teachoo.com
Activity to Show Gases can be Compressed but Solids and Liquids cannot Can You Compress Air Into A Liquid When you compress air, you force its molecules into a smaller space. Can you compress air into a liquid? If all the balls are bouncing really quickly, you would need additional space. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas. Can You Compress Air Into A Liquid.
From studypositivity.z21.web.core.windows.net
Why Can Gases Be Compressed Can You Compress Air Into A Liquid If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. If you compress liquid, it doesn't have anywhere to go. It only seems like it because you can see gases be compressed, but you can't see it in liquids. Think of. Can You Compress Air Into A Liquid.
From www.nagwa.com
Question Video Calculating the Work Done to Compress an Ideal Gas Nagwa Can You Compress Air Into A Liquid Bulk modulus is a measure of a substance's resistance to uniform compression. When you compress air, you force its molecules into a smaller space. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. This involves compressing air using a. It only seems like it because you can see gases be compressed,. Can You Compress Air Into A Liquid.
From fluidflow.com
Compressed Air Fluid Flow Can You Compress Air Into A Liquid When you compress air, you force its molecules into a smaller space. It only seems like it because you can see gases be compressed, but you can't see it in liquids. Think of it as a large room of bouncing balls. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. This. Can You Compress Air Into A Liquid.
From c1sinc.com
Basics of Compressed Air System Design and Troubleshooting Part Two Can You Compress Air Into A Liquid Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. If all the balls are bouncing really quickly, you would need additional space. Bulk modulus is a measure of a substance's. Can You Compress Air Into A Liquid.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science Can You Compress Air Into A Liquid Think of it as a large room of bouncing balls. This involves compressing air using a. This increased density makes the molecules move faster, increasing. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. Bulk modulus is a measure of a substance's resistance to uniform compression. When you compress air, you. Can You Compress Air Into A Liquid.
From www.youtube.com
Can solids, liquids and gases be compressed? Grade Three Science Can You Compress Air Into A Liquid It only seems like it because you can see gases be compressed, but you can't see it in liquids. Bulk modulus is a measure of a substance's resistance to uniform compression. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. If you compress liquid, it doesn't have anywhere to go. Can. Can You Compress Air Into A Liquid.
From www.air-compressor-guide.com
The problem. Can You Compress Air Into A Liquid If you compress liquid, it doesn't have anywhere to go. Can you compress air into a liquid? Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. Think of it as a large room of bouncing balls. If all the balls are bouncing really quickly, you would need additional. Can You Compress Air Into A Liquid.
From www.teachoo.com
Pressure Exerted by Liquids and Gases Class 8 Science Notes Can You Compress Air Into A Liquid If all the balls are bouncing really quickly, you would need additional space. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. When you compress air, you force its molecules into a smaller space. Think of it as a large room of bouncing balls. If you compress liquid, it doesn't have. Can You Compress Air Into A Liquid.
From www.air-compressor-guide.com
Actual = 68 g/m³ Can You Compress Air Into A Liquid If you compress liquid, it doesn't have anywhere to go. Can you compress air into a liquid? It only seems like it because you can see gases be compressed, but you can't see it in liquids. This involves compressing air using a. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that. Can You Compress Air Into A Liquid.
From www.youtube.com
Can You Compress Water With Hydraulic Press Using 2000 Bars / 29 000 Can You Compress Air Into A Liquid If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. Think of it as a large room of bouncing balls. Can you compress air into a liquid? It only seems like it because you can see gases be compressed, but you. Can You Compress Air Into A Liquid.
From tuongotchinsu.net
Can Air Be Compressed Into A Solid? Exploring The Possibilities Can You Compress Air Into A Liquid If you compress liquid, it doesn't have anywhere to go. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. This increased density makes the molecules move faster, increasing. Can you compress air into a liquid? Think of it as a large room of bouncing balls. If all the balls are bouncing. Can You Compress Air Into A Liquid.
From www.youtube.com
How To Use a Can of Compressed Air Correctly YouTube Can You Compress Air Into A Liquid Bulk modulus is a measure of a substance's resistance to uniform compression. Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. If all the balls are bouncing really quickly, you would need additional space. Think of it as a large room of bouncing balls. This involves compressing air. Can You Compress Air Into A Liquid.
From www.youtube.com
Thermodynamics COMPRESSED Liquid Properties in 6 Minutes! YouTube Can You Compress Air Into A Liquid Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. If you compress liquid, it doesn't have anywhere to go. If all the balls are bouncing really quickly, you would need additional space. Bulk modulus is a measure of a substance's resistance to uniform compression. Can you compress air. Can You Compress Air Into A Liquid.
From sansona.github.io
Solids, Liquids, and Gases Can You Compress Air Into A Liquid Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. Can you compress air into a liquid? This increased density makes the molecules move faster, increasing. Think of it as a large room of bouncing balls. If you compress liquid, it doesn't have anywhere to go. If you compress your gas enough. Can You Compress Air Into A Liquid.
From www.sciencefacts.net
Physics Page 7 of 19 Science Facts Can You Compress Air Into A Liquid Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. When you compress air, you force its molecules into a smaller space. It only seems like it because you can see gases be compressed, but you can't see it in liquids. Think of it as a large room of bouncing balls. If. Can You Compress Air Into A Liquid.
From www.youtube.com
Module 3 Compressed liquid YouTube Can You Compress Air Into A Liquid This involves compressing air using a. This increased density makes the molecules move faster, increasing. Think of it as a large room of bouncing balls. Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. It only seems like it because you can see gases be compressed, but you. Can You Compress Air Into A Liquid.
From www.youtube.com
Activity to show that gases can be compressed more easily than liquids Can You Compress Air Into A Liquid It only seems like it because you can see gases be compressed, but you can't see it in liquids. Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. Can you compress air into a liquid? Air is a mixture of nitrogen, oxygen and other gases and can be. Can You Compress Air Into A Liquid.
From www.youtube.com
How to Turn Air Into a Liquid YouTube Can You Compress Air Into A Liquid When you compress air, you force its molecules into a smaller space. Think of it as a large room of bouncing balls. This involves compressing air using a. It only seems like it because you can see gases be compressed, but you can't see it in liquids. If all the balls are bouncing really quickly, you would need additional space.. Can You Compress Air Into A Liquid.
From exolfelrd.blob.core.windows.net
Process Of Compressed Air Energy Storage at White blog Can You Compress Air Into A Liquid Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. This increased density makes the molecules move faster, increasing. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. This involves compressing air. Can You Compress Air Into A Liquid.
From www.youtube.com
Atlas Copco Compressors Chapter 2 What is compressed air? YouTube Can You Compress Air Into A Liquid Can you compress air into a liquid? This increased density makes the molecules move faster, increasing. Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. If all the balls are bouncing really quickly, you would need additional space. If you compress your gas enough (and lower the temperature), eventually the particles. Can You Compress Air Into A Liquid.
From www.slideserve.com
PPT Chapter 14.1 1 Key Concept Why is a gas easy to compress Can You Compress Air Into A Liquid Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. If all the balls are bouncing really quickly, you would need additional space. This involves compressing air using a. If you. Can You Compress Air Into A Liquid.
From www.youtube.com
Fluid Mechanics Introduction Compressibility of Fluids YouTube Can You Compress Air Into A Liquid Think of it as a large room of bouncing balls. It only seems like it because you can see gases be compressed, but you can't see it in liquids. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. If you. Can You Compress Air Into A Liquid.
From www.youtube.com
Freezing water with compressed air YouTube Can You Compress Air Into A Liquid If all the balls are bouncing really quickly, you would need additional space. Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. This increased density makes the molecules move faster, increasing. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough. Can You Compress Air Into A Liquid.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Can You Compress Air Into A Liquid Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns. It only seems like it because you can see gases. Can You Compress Air Into A Liquid.
From www.physicsforums.com
How to compute the energy needed to compress the water isothermally? Can You Compress Air Into A Liquid It only seems like it because you can see gases be compressed, but you can't see it in liquids. When you compress air, you force its molecules into a smaller space. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to attract each other, and then your gas turns.. Can You Compress Air Into A Liquid.
From www.sciencefacts.net
Are Liquids Compressible Can You Compress Air Into A Liquid It only seems like it because you can see gases be compressed, but you can't see it in liquids. Can you compress air into a liquid? When you compress air, you force its molecules into a smaller space. If all the balls are bouncing really quickly, you would need additional space. Bulk modulus is a measure of a substance's resistance. Can You Compress Air Into A Liquid.
From engineeringsales.com
Why Compressed Air Piping Size Matters ESA Can You Compress Air Into A Liquid Air is a mixture of nitrogen, oxygen and other gases and can be liquefied by cooling it to. If you compress liquid, it doesn't have anywhere to go. This involves compressing air using a. This increased density makes the molecules move faster, increasing. Can you compress air into a liquid? When you compress air, you force its molecules into a. Can You Compress Air Into A Liquid.
From www.yaclass.in
Compressibility of solids, liquids and gases — lesson. Science State Can You Compress Air Into A Liquid Bulk modulus is a measure of a substance's resistance to uniform compression. When you compress air, you force its molecules into a smaller space. Can you compress air into a liquid? This increased density makes the molecules move faster, increasing. Think of it as a large room of bouncing balls. If all the balls are bouncing really quickly, you would. Can You Compress Air Into A Liquid.
From www.mdpi.com
Thermo Free FullText Comprehensive Review of Compressed Air Energy Can You Compress Air Into A Liquid If all the balls are bouncing really quickly, you would need additional space. If you compress liquid, it doesn't have anywhere to go. This increased density makes the molecules move faster, increasing. Can you compress air into a liquid? Bulk modulus is a measure of a substance's resistance to uniform compression. When you compress air, you force its molecules into. Can You Compress Air Into A Liquid.
From www.youtube.com
Atlas Copco Compressors Chapter 18 What is a compressed air Can You Compress Air Into A Liquid This involves compressing air using a. Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. Bulk modulus is a measure of a substance's resistance to uniform compression. If you compress your gas enough (and lower the temperature), eventually the particles will come close enough that they start to. Can You Compress Air Into A Liquid.
From www.automation--expert.com
Thermal Analysis Solutions Active Air Compressed Cooling Can You Compress Air Into A Liquid Cooling the air as it leaves the compressor will take most of the moisture out before it gets into the piping. Bulk modulus is a measure of a substance's resistance to uniform compression. If you compress liquid, it doesn't have anywhere to go. This involves compressing air using a. Can you compress air into a liquid? This increased density makes. Can You Compress Air Into A Liquid.