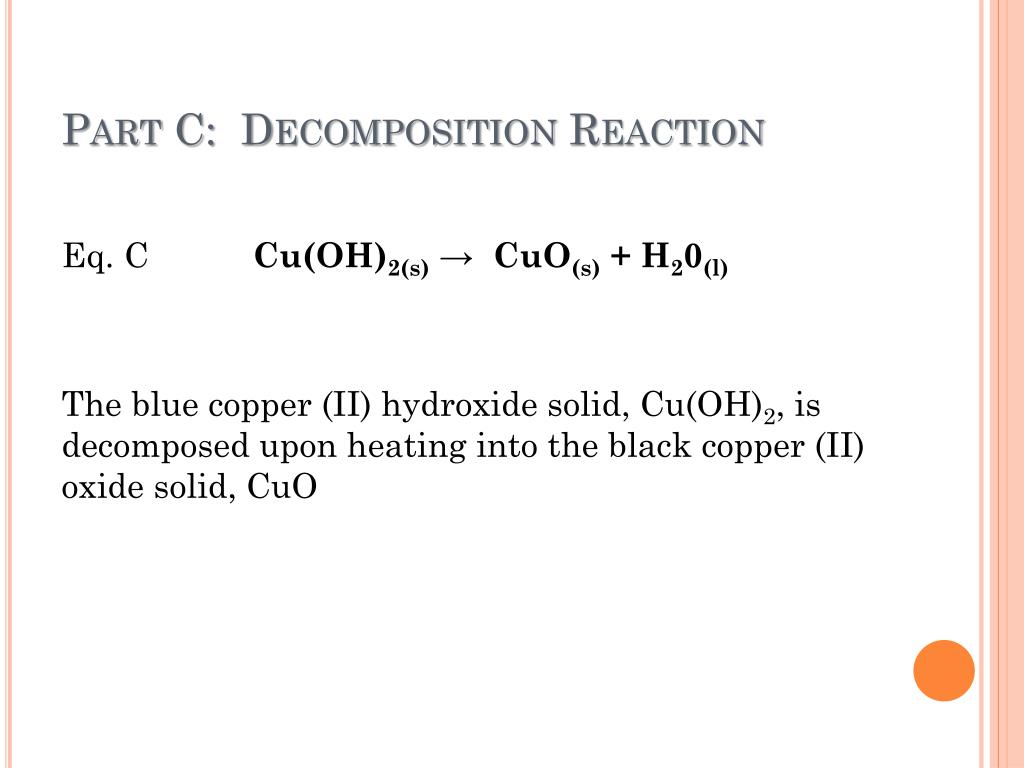
Copper 2 Oxide + H2So4 . Copper (ii) oxide, is a black solid, which,. What is the balanced equation for sulphuric acid in copper oxide? Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. 1 atom in reagents and 1 atom in products. Today we will be reacting. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. 2 atoms in reagents and 2 atoms in products. Mrs v is back in the lockdown lab with some acid reactions.
from www.slideserve.com
1 atom in reagents and 1 atom in products. Mrs v is back in the lockdown lab with some acid reactions. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. What is the balanced equation for sulphuric acid in copper oxide? 2 atoms in reagents and 2 atoms in products. Copper (ii) oxide, is a black solid, which,. Today we will be reacting.
PPT Chemical Reactions Copper Reactions PowerPoint Presentation, free download ID4272546
Copper 2 Oxide + H2So4 Today we will be reacting. What is the balanced equation for sulphuric acid in copper oxide? Today we will be reacting. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. 2 atoms in reagents and 2 atoms in products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. 1 atom in reagents and 1 atom in products. Copper (ii) oxide, is a black solid, which,. Mrs v is back in the lockdown lab with some acid reactions.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas YouTube Copper 2 Oxide + H2So4 Copper (ii) oxide, is a black solid, which,. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. Mrs v is back in the lockdown lab with some acid reactions. Today we will be reacting. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide. Copper 2 Oxide + H2So4.
From www.youtube.com
H2SO4 TÁC DỤNG VỚI Cu(OH)2 thinghiemhoahoc H2SO4 hoacobichngoc hoa11 YouTube Copper 2 Oxide + H2So4 What is the balanced equation for sulphuric acid in copper oxide? Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Today we will be reacting. 1 atom in reagents and 1 atom in products. Copper (ii) oxide, is a black solid, which,. Mixing copper oxide and sulphuric acid is an. Copper 2 Oxide + H2So4.
From www.semanticscholar.org
Figure 1 from Copper Extraction from Oxide Ore of Almalyk Mine by H2SO4 in Simulated Heap Copper 2 Oxide + H2So4 Copper (ii) oxide, is a black solid, which,. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Today we will be reacting. 2 atoms in reagents and 2 atoms in products. 1 atom in reagents and 1 atom in products. Mixing copper oxide and sulphuric acid is an experiment involving. Copper 2 Oxide + H2So4.
From www.sciencephoto.com
Copper (II) oxide reacts with sulfuric acid Stock Image C036/3130 Science Photo Library Copper 2 Oxide + H2So4 What is the balanced equation for sulphuric acid in copper oxide? Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. Mrs v is back in the lockdown lab with some acid reactions. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is. Copper 2 Oxide + H2So4.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint Copper 2 Oxide + H2So4 What is the balanced equation for sulphuric acid in copper oxide? Today we will be reacting. Mrs v is back in the lockdown lab with some acid reactions. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal. Copper 2 Oxide + H2So4.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation, free download ID4272546 Copper 2 Oxide + H2So4 Today we will be reacting. Mrs v is back in the lockdown lab with some acid reactions. Copper (ii) oxide, is a black solid, which,. What is the balanced equation for sulphuric acid in copper oxide? Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a. Copper 2 Oxide + H2So4.
From www.abmstore.my
Copper 2 Oxide Systerm (500g) CAS No 1317380 Kuprum II Oksida CuO AbmStore.my Copper 2 Oxide + H2So4 Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. Copper (ii) oxide, is a black solid, which,. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Mrs v is back in. Copper 2 Oxide + H2So4.
From www.youtube.com
Cu + H2SO4 (Copper + Sulfuric acid) YouTube Copper 2 Oxide + H2So4 Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Today we will be reacting. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. 1 atom in reagents and 1 atom in products. Copper (ii) oxide, is a black. Copper 2 Oxide + H2So4.
From www.youtube.com
How to Balance Cu + H2SO4 → CuSO4 + SO2 + H2O Chemical equation Cu+H2SO4=CuSO4+SO2+H2O YouTube Copper 2 Oxide + H2So4 2 atoms in reagents and 2 atoms in products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Copper (ii) oxide, is a black solid, which,. Today we will be reacting. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to. Copper 2 Oxide + H2So4.
From fineartamerica.com
Copper (ii) Oxide Photograph by Martyn F. Chillmaid/science Photo Library Fine Art America Copper 2 Oxide + H2So4 2 atoms in reagents and 2 atoms in products. Mrs v is back in the lockdown lab with some acid reactions. What is the balanced equation for sulphuric acid in copper oxide? Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. Today we will be reacting. Solved and. Copper 2 Oxide + H2So4.
From www.youtube.com
How to Write the Net Ionic Equation for KOH + H2SO4 = K2SO4 + H2O YouTube Copper 2 Oxide + H2So4 2 atoms in reagents and 2 atoms in products. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. What is the balanced equation for sulphuric acid in copper oxide? Copper (ii) oxide, is a black solid, which,. Mrs v is back in the lockdown lab with some acid reactions. Copper(ii). Copper 2 Oxide + H2So4.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation, free download ID35476 Copper 2 Oxide + H2So4 Mrs v is back in the lockdown lab with some acid reactions. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. What is the balanced equation for sulphuric acid in copper oxide? Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react. Copper 2 Oxide + H2So4.
From www.youtube.com
Adding H2SO4 to CuO precipitate YouTube Copper 2 Oxide + H2So4 2 atoms in reagents and 2 atoms in products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. Copper (ii) oxide, is. Copper 2 Oxide + H2So4.
From www.toppr.com
Balance the following reactions Cu + H2SO4→ CuSO4 + SO2↑ + H2O Copper 2 Oxide + H2So4 Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. 1 atom in reagents and 1 atom in products. What is the balanced equation for sulphuric acid in copper oxide? Mrs v is back in the lockdown lab with some acid reactions. Copper (ii) oxide, is a black solid,. Copper 2 Oxide + H2So4.
From www.flinnsci.ca
Copper(II) Oxide, Powder, 100 g Flinn Scientific Copper 2 Oxide + H2So4 Mrs v is back in the lockdown lab with some acid reactions. Today we will be reacting. 1 atom in reagents and 1 atom in products. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. 2 atoms in reagents and 2 atoms in products. What is the balanced equation for. Copper 2 Oxide + H2So4.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint Copper 2 Oxide + H2So4 What is the balanced equation for sulphuric acid in copper oxide? Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Copper (ii) oxide, is a black solid, which,. Today we will be reacting. 1 atom in reagents and 1 atom in products. Copper(ii). Copper 2 Oxide + H2So4.
From www.sciencephoto.com
Copper (II) oxide reacting with sulphuric acid Stock Image C052/5207 Science Photo Library Copper 2 Oxide + H2So4 What is the balanced equation for sulphuric acid in copper oxide? Mrs v is back in the lockdown lab with some acid reactions. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with. Copper 2 Oxide + H2So4.
From blog.thepipingmart.com
Copper Oxidation States and Colors Copper 2 Oxide + H2So4 1 atom in reagents and 1 atom in products. 2 atoms in reagents and 2 atoms in products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o. Copper 2 Oxide + H2So4.
From www.semanticscholar.org
Figure 1 from Copper Extraction from Oxide Ore of Almalyk Mine by H2SO4 in Simulated Heap Copper 2 Oxide + H2So4 1 atom in reagents and 1 atom in products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Mrs v is back in the lockdown lab with some acid reactions. 2 atoms in reagents and 2 atoms in products. Solved and balanced chemical. Copper 2 Oxide + H2So4.
From www.numerade.com
SOLVED Which of the following shows the balanced equation for Copper(II) oxide with sulfuric Copper 2 Oxide + H2So4 1 atom in reagents and 1 atom in products. What is the balanced equation for sulphuric acid in copper oxide? Today we will be reacting. 2 atoms in reagents and 2 atoms in products. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Copper(ii) oxide, a black solid, and colourless. Copper 2 Oxide + H2So4.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation, free download ID35476 Copper 2 Oxide + H2So4 Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. Mrs v is back in the lockdown lab with some acid reactions. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Copper. Copper 2 Oxide + H2So4.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Finding the formula of copper(II) oxide Experiment RSC Education Copper 2 Oxide + H2So4 Copper (ii) oxide, is a black solid, which,. 1 atom in reagents and 1 atom in products. Mrs v is back in the lockdown lab with some acid reactions. Today we will be reacting. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. 2 atoms in reagents and 2 atoms. Copper 2 Oxide + H2So4.
From www.numerade.com
SOLVED CuO (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l) When 5.00 g of copper oxide reacts with 1.00 Copper 2 Oxide + H2So4 1 atom in reagents and 1 atom in products. Copper (ii) oxide, is a black solid, which,. 2 atoms in reagents and 2 atoms in products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Solved and balanced chemical equation 2 h2so4 +. Copper 2 Oxide + H2So4.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 + H2SO4 = CuSO4 + H2O YouTube Copper 2 Oxide + H2So4 Mrs v is back in the lockdown lab with some acid reactions. Copper (ii) oxide, is a black solid, which,. What is the balanced equation for sulphuric acid in copper oxide? 1 atom in reagents and 1 atom in products. 2 atoms in reagents and 2 atoms in products. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react. Copper 2 Oxide + H2So4.
From espanol.noahchemicals.com
4 usos del óxido cúprico (óxido de cobre II) Noah Chemicals Copper 2 Oxide + H2So4 Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. 2 atoms in reagents and 2 atoms in products. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. What is the balanced equation for. Copper 2 Oxide + H2So4.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Chemistry of Copper PowerPoint Copper 2 Oxide + H2So4 Mrs v is back in the lockdown lab with some acid reactions. What is the balanced equation for sulphuric acid in copper oxide? Copper (ii) oxide, is a black solid, which,. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. 1 atom in reagents and 1 atom in products. Today. Copper 2 Oxide + H2So4.
From www.numerade.com
SOLVED 1) What would happen when a) Copper oxide reacts with dilute H2SO4. b) Magnesium reacts Copper 2 Oxide + H2So4 Today we will be reacting. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. What is the balanced equation for sulphuric acid in copper oxide? Mrs v is back in the lockdown lab with some acid reactions. Copper(ii) oxide, a black solid, and. Copper 2 Oxide + H2So4.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation, free download ID35476 Copper 2 Oxide + H2So4 What is the balanced equation for sulphuric acid in copper oxide? Today we will be reacting. 2 atoms in reagents and 2 atoms in products. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Copper (ii) oxide, is a black solid, which,. Mrs v is back in the lockdown lab. Copper 2 Oxide + H2So4.
From slideplayer.com
Discuss in pairs What is an isotope?. ppt download Copper 2 Oxide + H2So4 2 atoms in reagents and 2 atoms in products. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. Today we will be reacting. Mrs v is back in the lockdown lab with some acid reactions. What is the balanced equation for sulphuric acid in copper oxide? Mixing copper. Copper 2 Oxide + H2So4.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation, free download ID4272546 Copper 2 Oxide + H2So4 2 atoms in reagents and 2 atoms in products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. 1 atom in reagents and 1 atom in products. What is the balanced equation for sulphuric acid in copper oxide? Copper (ii) oxide, is a. Copper 2 Oxide + H2So4.
From www.sciencephoto.com
Two copper oxide reactions Stock Image A500/0465 Science Photo Library Copper 2 Oxide + H2So4 Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. 2 atoms in reagents and 2 atoms in products. Mixing copper oxide and sulphuric acid is an experiment involving an. Copper 2 Oxide + H2So4.
From www.youtube.com
How to Balance CuO + H2SO4 = CuSO4 + H2O YouTube Copper 2 Oxide + H2So4 Mrs v is back in the lockdown lab with some acid reactions. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. Copper (ii) oxide, is a black solid, which,. Today we will be reacting. Solved and balanced chemical equation 2 h2so4 + cu. Copper 2 Oxide + H2So4.
From www.youtube.com
80 CuO + H2SO4 Copper oxide + Sulfuric acid💚 YouTube Copper 2 Oxide + H2So4 Copper (ii) oxide, is a black solid, which,. Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Today we will be reacting. What is the balanced equation for sulphuric acid in copper oxide? 1 atom in reagents and 1 atom in products. 2 atoms in reagents and 2 atoms in. Copper 2 Oxide + H2So4.
From www.youtube.com
How to Write the Net Ionic Equation for Cu(OH)2 + H2SO4 = CuSO4 + H2O YouTube Copper 2 Oxide + H2So4 Solved and balanced chemical equation 2 h2so4 + cu → cuso4 + so2 + 2 h2o with completed products. Copper (ii) oxide, is a black solid, which,. What is the balanced equation for sulphuric acid in copper oxide? 1 atom in reagents and 1 atom in products. Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal. Copper 2 Oxide + H2So4.
From www.youtube.com
Copper Sulfate and Sodium Hydroxide YouTube Copper 2 Oxide + H2So4 Copper(ii) oxide, a black solid, and colourless dilute sulfuric acid react to produce copper(ii) sulfate, giving a characteristic blue colour to. 2 atoms in reagents and 2 atoms in products. What is the balanced equation for sulphuric acid in copper oxide? Mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a. Copper 2 Oxide + H2So4.