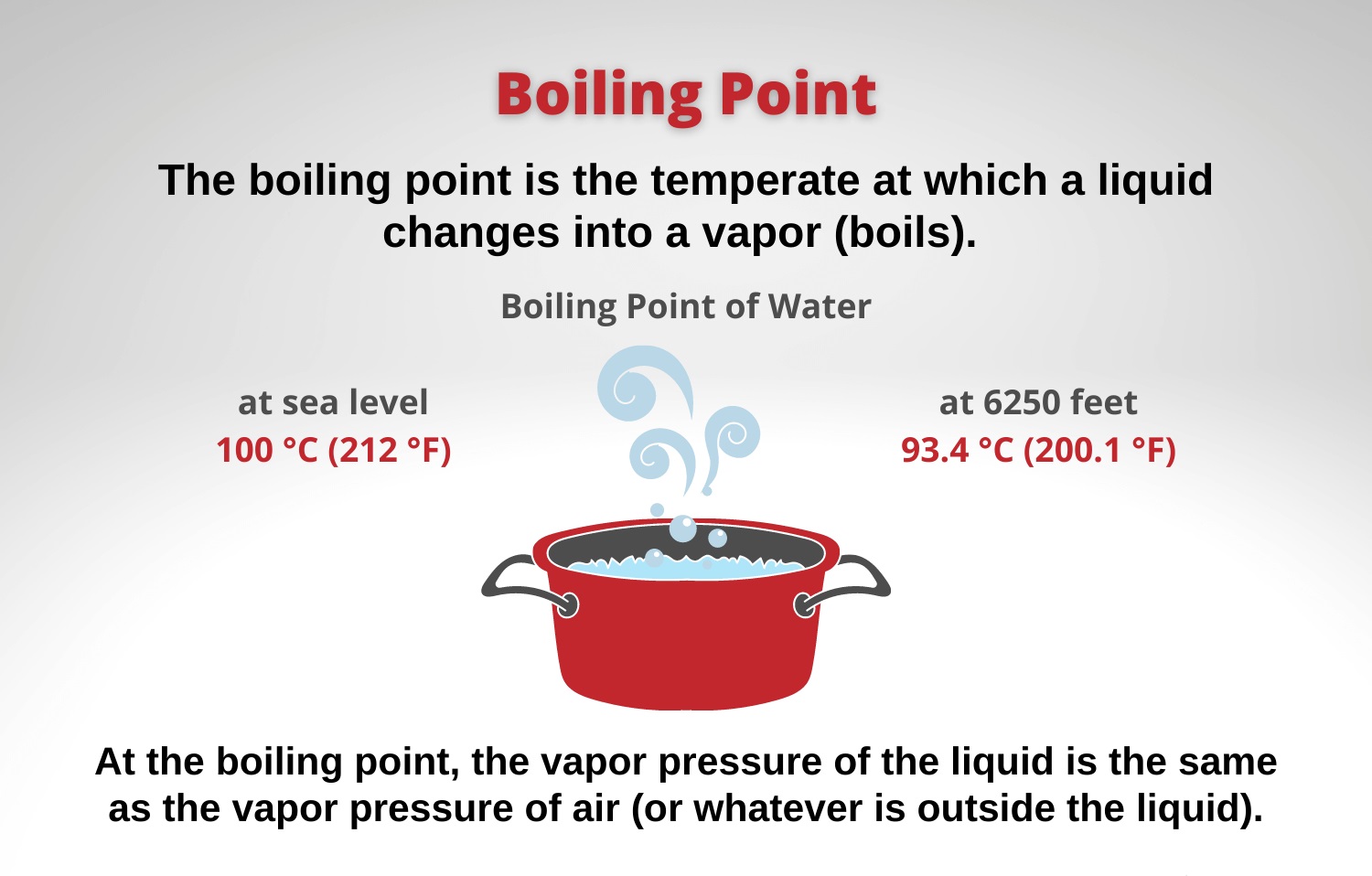
Does Boiling Reduce Hardness Of Water . The answer is the water reaches its boiling point temperature and stays there. However, this boiling point decreases at higher. Temporary hardness is removed by boiling the water. The boiling point of water is 100°c. When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. And other carbonates and bicarbonates),. To quickly soften the hard water in your. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. What is an example of liquid that boiling. The boiling of temporary hard water forms a layer of. In this case, the hardness in water can be removed by boiling the water. This may coat the heating. The temperature at which water boils isn’t the same everywhere. At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). What is an example of liquid that boils a temperature higher than the boiling point of water?
from facts.net
At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). The temperature at which water boils isn’t the same everywhere. The boiling of temporary hard water forms a layer of. Temporary hardness is removed by boiling the water. Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: What is an example of liquid that boiling. And other carbonates and bicarbonates),. The boiling point of water is 100°c. To quickly soften the hard water in your. What is an example of liquid that boils a temperature higher than the boiling point of water?
20 Fascinating Facts About Boiling Point
Does Boiling Reduce Hardness Of Water When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. In this case, the hardness in water can be removed by boiling the water. The answer is the water reaches its boiling point temperature and stays there. When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. The boiling point of water is 100°c. However, this boiling point decreases at higher. And other carbonates and bicarbonates),. The temperature at which water boils isn’t the same everywhere. Temporary hardness is removed by boiling the water. To quickly soften the hard water in your. At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). The boiling of temporary hard water forms a layer of. What is an example of liquid that boils a temperature higher than the boiling point of water? What is an example of liquid that boiling. This may coat the heating.
From www.livingwhole.com.au
Does boiling water remove fluoride? The Answer is No Living Whole Does Boiling Reduce Hardness Of Water Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: What is an example of liquid that boils a temperature higher than the boiling point of water? When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. However, this. Does Boiling Reduce Hardness Of Water.
From www.youtube.com
How To Boil Water (3 Types of Boiled Water) How To Boil Water How To Boil Water For Steam Does Boiling Reduce Hardness Of Water And other carbonates and bicarbonates),. Temporary hardness is removed by boiling the water. In this case, the hardness in water can be removed by boiling the water. The boiling point of water is 100°c. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. At sea level, pure water boils. Does Boiling Reduce Hardness Of Water.
From www.youtube.com
How Temporary Hardness Is Different from Permanent Hardness Water Hardness Explained YouTube Does Boiling Reduce Hardness Of Water The temperature at which water boils isn’t the same everywhere. The boiling point of water is 100°c. At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). In this case, the hardness in water can be removed by boiling the water. Temporary hardness is removed by boiling the water. What is an example of liquid that boils. Does Boiling Reduce Hardness Of Water.
From netsolwater.com
How to remove Hardness from wastewater Does Boiling Reduce Hardness Of Water And other carbonates and bicarbonates),. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. Temporary hardness is removed by boiling the water. In this case, the hardness in water can be removed by boiling the water. However, this boiling point decreases at higher. The answer is the water reaches. Does Boiling Reduce Hardness Of Water.
From crescentcity-fl.com
Boil Water Notices Crescent City, Florida Does Boiling Reduce Hardness Of Water To quickly soften the hard water in your. What is an example of liquid that boils a temperature higher than the boiling point of water? This may coat the heating. And other carbonates and bicarbonates),. What is an example of liquid that boiling. The boiling point of water is 100°c. The temperature at which water boils isn’t the same everywhere.. Does Boiling Reduce Hardness Of Water.
From www.youtube.com
Testing Water for Hardness Boiling Point YouTube Does Boiling Reduce Hardness Of Water However, this boiling point decreases at higher. Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: And other carbonates and bicarbonates),. The temperature at which water boils isn’t the same everywhere. In this case, the hardness in water can be removed by boiling the water. Temporary hardness. Does Boiling Reduce Hardness Of Water.
From purewaterblog.com
Can Water Be Too Soft? Homeowner’s Guide to Hard Water Water Treatment Does Boiling Reduce Hardness Of Water At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). However, this boiling point decreases at higher. The answer is the water reaches its boiling point temperature and stays there. When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. The temperature at which water boils isn’t the. Does Boiling Reduce Hardness Of Water.
From melscience.com
Water hardness MEL Chemistry Does Boiling Reduce Hardness Of Water The answer is the water reaches its boiling point temperature and stays there. The boiling of temporary hard water forms a layer of. However, this boiling point decreases at higher. The boiling point of water is 100°c. What is an example of liquid that boils a temperature higher than the boiling point of water? The temperature at which water boils. Does Boiling Reduce Hardness Of Water.
From www.mdpi.com
Water Free FullText Removal of Hardness from Water Samples by a Carbonation Process with a Does Boiling Reduce Hardness Of Water The answer is the water reaches its boiling point temperature and stays there. The boiling point of water is 100°c. When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. However, this boiling point decreases at higher. In this case, the hardness in water can be removed by boiling the water.. Does Boiling Reduce Hardness Of Water.
From purewaterblog.com
Does Boiling Hard Water Make It Soft? Water Treatment Does Boiling Reduce Hardness Of Water The boiling point of water is 100°c. To quickly soften the hard water in your. What is an example of liquid that boils a temperature higher than the boiling point of water? Temporary hardness is removed by boiling the water. The answer is the water reaches its boiling point temperature and stays there. To soften small batches of hard water,. Does Boiling Reduce Hardness Of Water.
From sciencenotes.org
Does Boiling Water Keep Getting Hotter? Does Boiling Reduce Hardness Of Water To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. Temporary hardness is removed by boiling the water. This may coat the heating. The boiling point of water is 100°c. What is an example of liquid that boiling. In this case, the hardness in water can be removed by boiling. Does Boiling Reduce Hardness Of Water.
From www.slideserve.com
PPT Water hardness & Special treatment PowerPoint Presentation ID6240851 Does Boiling Reduce Hardness Of Water When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. And other carbonates and bicarbonates),. This may coat the heating. Boiling water will not reduce hardness of water, whereas heating hard. Does Boiling Reduce Hardness Of Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? Does Boiling Reduce Hardness Of Water In this case, the hardness in water can be removed by boiling the water. However, this boiling point decreases at higher. What is an example of liquid that boiling. What is an example of liquid that boils a temperature higher than the boiling point of water? At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). Temporary. Does Boiling Reduce Hardness Of Water.
From waterfilterguru.com
Does Boiling Hard Water Make It Soft? Does Boiling Reduce Hardness Of Water To quickly soften the hard water in your. And other carbonates and bicarbonates),. At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). This may coat the heating. When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. The temperature at which water boils isn’t the same everywhere.. Does Boiling Reduce Hardness Of Water.
From www.youtube.com
How to determine hardness of water by EDTA method? (Procedure and problems on hardness) YouTube Does Boiling Reduce Hardness Of Water The answer is the water reaches its boiling point temperature and stays there. At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). To quickly soften the hard water in your. This may coat the heating. However, this boiling point decreases at higher. Temporary hardness is removed by boiling the water. The boiling point of water is. Does Boiling Reduce Hardness Of Water.
From www.scienceabc.com
Why Does Water Boil Quickly At High Altitudes? » ScienceABC Does Boiling Reduce Hardness Of Water Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. What is an example of liquid that boils a temperature higher than the boiling point of water? What. Does Boiling Reduce Hardness Of Water.
From www.youtube.com
Softening of Water Method for removing hardness from water How temporary hardness removed Does Boiling Reduce Hardness Of Water What is an example of liquid that boiling. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. In this case, the hardness in water can be removed by boiling the water. What is an example of liquid that boils a temperature higher than the boiling point of water? When. Does Boiling Reduce Hardness Of Water.
From slideplayer.com
Water H2O What do the students think of when they see this picture? ppt download Does Boiling Reduce Hardness Of Water To quickly soften the hard water in your. When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. What is an example of liquid that boiling. The temperature at which water boils isn’t the same everywhere. To soften small batches of hard water, boiling your water is the quickest method, but. Does Boiling Reduce Hardness Of Water.
From www.slideserve.com
PPT Water Hardness PowerPoint Presentation, free download ID2529744 Does Boiling Reduce Hardness Of Water What is an example of liquid that boiling. At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. This may coat the heating. In this case, the hardness in water can be removed by boiling the water. However,. Does Boiling Reduce Hardness Of Water.
From www.youtube.com
removal of permanent hardness of water by washing soda method YouTube Does Boiling Reduce Hardness Of Water When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. What is an example of liquid that boils a temperature higher than the boiling point of water? However, this boiling point. Does Boiling Reduce Hardness Of Water.
From ddcountermeasures.com
3 Ways To Treat Hard Water At Home Without Breaking The Bank Does Boiling Reduce Hardness Of Water Temporary hardness is removed by boiling the water. The answer is the water reaches its boiling point temperature and stays there. To quickly soften the hard water in your. Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: What is an example of liquid that boiling. The. Does Boiling Reduce Hardness Of Water.
From www.slideserve.com
PPT WATER POLLUTION PowerPoint Presentation, free download ID8934648 Does Boiling Reduce Hardness Of Water This may coat the heating. The boiling of temporary hard water forms a layer of. What is an example of liquid that boils a temperature higher than the boiling point of water? At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). Temporary hardness is removed by boiling the water. To soften small batches of hard water,. Does Boiling Reduce Hardness Of Water.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy Does Boiling Reduce Hardness Of Water However, this boiling point decreases at higher. The boiling point of water is 100°c. This may coat the heating. Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: What is an example of liquid that boils a temperature higher than the boiling point of water? The answer. Does Boiling Reduce Hardness Of Water.
From engineeringstuff.co.in
What is Boiling point Engineeringstuff Does Boiling Reduce Hardness Of Water However, this boiling point decreases at higher. In this case, the hardness in water can be removed by boiling the water. What is an example of liquid that boiling. The boiling point of water is 100°c. To quickly soften the hard water in your. Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point. Does Boiling Reduce Hardness Of Water.
From facts.net
20 Fascinating Facts About Boiling Point Does Boiling Reduce Hardness Of Water The boiling of temporary hard water forms a layer of. Temporary hardness is removed by boiling the water. What is an example of liquid that boiling. To quickly soften the hard water in your. This may coat the heating. The answer is the water reaches its boiling point temperature and stays there. At sea level, pure water boils at 100. Does Boiling Reduce Hardness Of Water.
From hydrojourney.com
Boiling Hard Water Quick Fix Or Permanent Solution? Hydro Journey Does Boiling Reduce Hardness Of Water The boiling point of water is 100°c. However, this boiling point decreases at higher. Temporary hardness is removed by boiling the water. The answer is the water reaches its boiling point temperature and stays there. This may coat the heating. Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of. Does Boiling Reduce Hardness Of Water.
From marina-kcasey.blogspot.com
Describe the Sound of Boiling Water Does Boiling Reduce Hardness Of Water Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: To quickly soften the hard water in your. What is an example of liquid that boils a temperature higher than the boiling point of water? The answer is the water reaches its boiling point temperature and stays there.. Does Boiling Reduce Hardness Of Water.
From www.seriouseats.com
Everything You Ever Wanted to Know (Plus More!) About Boiling Water The Food Lab Does Boiling Reduce Hardness Of Water What is an example of liquid that boils a temperature higher than the boiling point of water? And other carbonates and bicarbonates),. What is an example of liquid that boiling. However, this boiling point decreases at higher. Temporary hardness is removed by boiling the water. This may coat the heating. The boiling point of water is 100°c. At sea level,. Does Boiling Reduce Hardness Of Water.
From www.slideserve.com
PPT Water Treatment Basics PowerPoint Presentation, free download ID3356319 Does Boiling Reduce Hardness Of Water When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. The boiling point of water is 100°c. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. The answer is the water reaches its boiling point temperature and stays there. At. Does Boiling Reduce Hardness Of Water.
From hikingmastery.com
Does Boiling Water Purify It Basic Facts and Useful Does Boiling Reduce Hardness Of Water Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: Temporary hardness is removed by boiling the water. And other carbonates and bicarbonates),. To quickly soften the hard water in your. The boiling point of water is 100°c. In this case, the hardness in water can be removed. Does Boiling Reduce Hardness Of Water.
From www.youtube.com
Methods of Removal of Temporary Hardness Water By Boiling and Clark's Method YouTube Does Boiling Reduce Hardness Of Water Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of $caco_3$ (edit: The answer is the water reaches its boiling point temperature and stays there. The temperature at which water boils isn’t the same everywhere. The boiling of temporary hard water forms a layer of. What is an example of. Does Boiling Reduce Hardness Of Water.
From apiqohofrr.blogspot.com
How Do You Know If Water Is Boiling Learn about how water purity and hardness affects its Does Boiling Reduce Hardness Of Water This may coat the heating. To quickly soften the hard water in your. At sea level, pure water boils at 100 degrees celsius (212 degrees fahrenheit). When we boil water, the soluble salts of mg (hco 3) 2 are converted to mg (oh) 2, which. The boiling point of water is 100°c. What is an example of liquid that boiling.. Does Boiling Reduce Hardness Of Water.
From www.slideshare.net
water hardness Does Boiling Reduce Hardness Of Water However, this boiling point decreases at higher. In this case, the hardness in water can be removed by boiling the water. The boiling point of water is 100°c. And other carbonates and bicarbonates),. To quickly soften the hard water in your. Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility. Does Boiling Reduce Hardness Of Water.
From slideplayer.com
Water Treatment. ppt download Does Boiling Reduce Hardness Of Water To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. In this case, the hardness in water can be removed by boiling the water. The boiling of temporary hard water forms a layer of. Temporary hardness is removed by boiling the water. What is an example of liquid that boils. Does Boiling Reduce Hardness Of Water.
From www.slideshare.net
Determination of hardness of water Does Boiling Reduce Hardness Of Water However, this boiling point decreases at higher. What is an example of liquid that boiling. To soften small batches of hard water, boiling your water is the quickest method, but it only addresses temporary hardness. This may coat the heating. Boiling water will not reduce hardness of water, whereas heating hard water to the boiling point will reduce solubility of. Does Boiling Reduce Hardness Of Water.