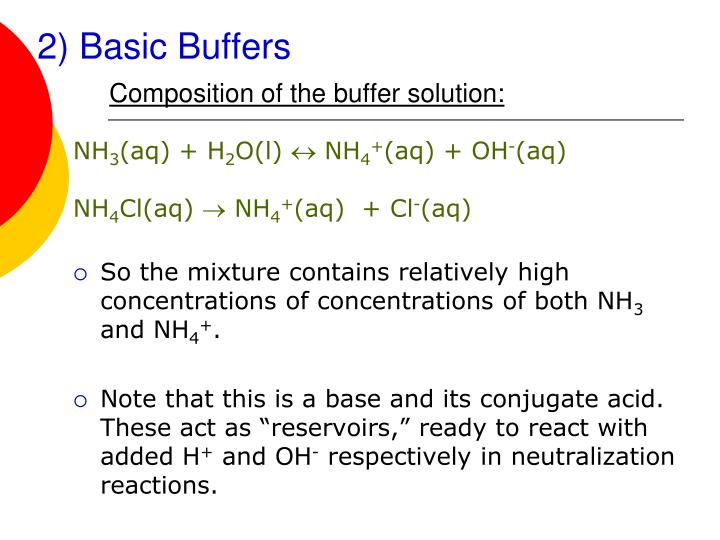
What Does A Buffer Do In Biology . Biological buffers are organic substances that help regulate the ph level in organisms. In this way, a biological buffer. Buffers are important in biological systems because of their ability to maintain. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. In other words, a buffer is an aqueous solution. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Four important generalizations about buffers. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. They act by neutralizing excess hydrogen ions, thereby maintaining the. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph.
from www.slideserve.com
They act by neutralizing excess hydrogen ions, thereby maintaining the. In this way, a biological buffer. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Buffers are important in biological systems because of their ability to maintain. Four important generalizations about buffers.
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504
What Does A Buffer Do In Biology In other words, a buffer is an aqueous solution. Four important generalizations about buffers. In other words, a buffer is an aqueous solution. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. Biological buffers are organic substances that help regulate the ph level in organisms. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. They act by neutralizing excess hydrogen ions, thereby maintaining the. In this way, a biological buffer. Buffers are important in biological systems because of their ability to maintain. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system.
From www.tffn.net
What is a Buffer in Science? Exploring the Role of Buffers in Chemistry and Biology The What Does A Buffer Do In Biology Biological buffers are organic substances that help regulate the ph level in organisms. In other words, a buffer is an aqueous solution. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. They act by neutralizing excess hydrogen ions, thereby maintaining the. Four important generalizations about buffers. Buffers are important in. What Does A Buffer Do In Biology.
From www.youtube.com
Introduction to buffers Water, acids, and bases Biology Khan Academy YouTube What Does A Buffer Do In Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In this way, a biological buffer. Four important generalizations about buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are important in biological systems because of their ability to maintain. The most relevant systems for biology are the carbonic. What Does A Buffer Do In Biology.
From www.youtube.com
Role of buffers in biological system YouTube What Does A Buffer Do In Biology Four important generalizations about buffers. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. Buffers are important in biological systems because of their ability to maintain. Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is. What Does A Buffer Do In Biology.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance Buffers in What Does A Buffer Do In Biology In this way, a biological buffer. Four important generalizations about buffers. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In other words, a buffer is an aqueous solution. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is. What Does A Buffer Do In Biology.
From deporecipe.co
10x Protein Running Buffer Recipe Deporecipe.co What Does A Buffer Do In Biology Buffers are important in biological systems because of their ability to maintain. Biological buffers are organic substances that help regulate the ph level in organisms. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate. What Does A Buffer Do In Biology.
From www.brainkart.com
How do buffers work? What Does A Buffer Do In Biology The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. A buffer is a solution containing either a weak. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 What Does A Buffer Do In Biology They act by neutralizing excess hydrogen ions, thereby maintaining the. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Biological buffers are organic substances that help regulate the ph level in organisms. Four important generalizations about buffers. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Biology Chapter 6 PowerPoint Presentation, free download ID2514063 What Does A Buffer Do In Biology A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Four important generalizations about buffers. In this way, a biological buffer. In other words,. What Does A Buffer Do In Biology.
From bitesizebio.com
How Do Buffers Work? An Easy Explaination For Biologists What Does A Buffer Do In Biology They act by neutralizing excess hydrogen ions, thereby maintaining the. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. In this way, a. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT pH and Buffers PowerPoint Presentation, free download ID2948821 What Does A Buffer Do In Biology In this way, a biological buffer. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are important in biological systems because of their ability to maintain. Four important generalizations about buffers. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph.. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Biological Buffers PowerPoint Presentation, free download ID3105280 What Does A Buffer Do In Biology Biological buffers are organic substances that help regulate the ph level in organisms. In other words, a buffer is an aqueous solution. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are important in biological systems because of their ability to maintain. Four important generalizations about buffers. In this way, a biological buffer. A buffer is a solution. What Does A Buffer Do In Biology.
From www.youtube.com
Buffers and pH Biology YouTube What Does A Buffer Do In Biology Buffers are important in biological systems because of their ability to maintain. Biological buffers are organic substances that help regulate the ph level in organisms. In other words, a buffer is an aqueous solution. In this way, a biological buffer. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Buffers. What Does A Buffer Do In Biology.
From www.youtube.com
Buffer action in the blood YouTube What Does A Buffer Do In Biology Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. In this way, a biological buffer. Buffers are important in biological systems because of their ability to maintain. The most. What Does A Buffer Do In Biology.
From www.youtube.com
WCLN Buffers Biology YouTube What Does A Buffer Do In Biology Biological buffers are organic substances that help regulate the ph level in organisms. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological buffer. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Buffers are important in biological systems because. What Does A Buffer Do In Biology.
From www.slideshare.net
Buffer system What Does A Buffer Do In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In this way, a biological buffer. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. In other words, a buffer is an aqueous solution. A buffer is a solution containing either a weak acid and. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT PART 4 Salt Hydrolysis and Buffer Solutions PowerPoint Presentation ID2529504 What Does A Buffer Do In Biology In other words, a buffer is an aqueous solution. Four important generalizations about buffers. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In this way, a biological buffer. Buffers are important. What Does A Buffer Do In Biology.
From www.expii.com
Buffers — Definition & Overview Expii What Does A Buffer Do In Biology In other words, a buffer is an aqueous solution. Biological buffers are organic substances that help regulate the ph level in organisms. In this way, a biological buffer. Buffers are important in biological systems because of their ability to maintain. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. The most. What Does A Buffer Do In Biology.
From www.vrogue.co
How Do Buffers Work An Easy Explaination For Biologis vrogue.co What Does A Buffer Do In Biology A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. Four important generalizations about buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Buffers are important. What Does A Buffer Do In Biology.
From facts.net
13 Enigmatic Facts About Buffer Capacity What Does A Buffer Do In Biology In other words, a buffer is an aqueous solution. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. The most relevant systems for biology are the carbonic. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Water & Buffers PowerPoint Presentation, free download ID3886735 What Does A Buffer Do In Biology Biological buffers are organic substances that help regulate the ph level in organisms. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. In this way, a biological buffer. A buffer is a solution containing either. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 What Does A Buffer Do In Biology Biological buffers are organic substances that help regulate the ph level in organisms. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Four important generalizations about buffers. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. A buffer is composed of. What Does A Buffer Do In Biology.
From id.pinterest.com
buffer solution , Acidic & basic buffer,weak acid +salt of weak acid with strong base, weak base What Does A Buffer Do In Biology A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. In this way, a biological buffer. Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Four important generalizations about buffers. In other words, a. What Does A Buffer Do In Biology.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its action. YouTube What Does A Buffer Do In Biology Buffers are important in biological systems because of their ability to maintain. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. In other words, a buffer is an aqueous solution. Biological buffers are organic substances that help regulate the ph level in organisms. In this way, a biological buffer. They act by neutralizing excess. What Does A Buffer Do In Biology.
From www.pinterest.com
Buffer Buffer solution, Easy science, Flashcards What Does A Buffer Do In Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Buffers are important in biological systems because of their ability to maintain. In other words, a buffer is an aqueous solution. Four important. What Does A Buffer Do In Biology.
From www.geeksforgeeks.org
Buffer Solution Definition, Types, Formula, Examples, and FAQs What Does A Buffer Do In Biology In this way, a biological buffer. Buffers are important in biological systems because of their ability to maintain. Biological buffers are organic substances that help regulate the ph level in organisms. In other words, a buffer is an aqueous solution. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A buffer is a solution. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 What Does A Buffer Do In Biology A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. Biological buffers are organic substances that help regulate the ph level in organisms. Buffers are important in biological systems because of their ability to maintain. In this way, a biological buffer. The most relevant systems for biology are the carbonic acid/carbonate. What Does A Buffer Do In Biology.
From www.leinco.com
Biological Buffers And pH Range Leinco Technologies What Does A Buffer Do In Biology Biological buffers are organic substances that help regulate the ph level in organisms. Buffers are important in biological systems because of their ability to maintain. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt,. What Does A Buffer Do In Biology.
From www.studocu.com
Biological Buffer Systems BIOLOGICAL BUFFER SYSTEMS Almost every biological process is Studocu What Does A Buffer Do In Biology A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in. What Does A Buffer Do In Biology.
From www.chemistrystudent.com
Buffer Solutions (ALevel) ChemistryStudent What Does A Buffer Do In Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. Four important generalizations about buffers. In this way, a biological buffer. They act by neutralizing excess hydrogen ions, thereby maintaining the. Biological buffers are organic substances that help regulate the ph level in organisms. In other words, a buffer is an aqueous. What Does A Buffer Do In Biology.
From slidetodoc.com
Regulation of Extracellular Fluid Osmolarity and Sodium Concentration What Does A Buffer Do In Biology A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. In other words, a buffer is an aqueous solution. They act by neutralizing excess. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Blood Gases, pH and Buffer system PowerPoint Presentation ID257615 What Does A Buffer Do In Biology Buffers are solutions that moderate ph changes when an acid or base is added to the buffer system. In other words, a buffer is an aqueous solution. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Presentation ID5857540 What Does A Buffer Do In Biology Biological buffers are organic substances that help regulate the ph level in organisms. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. Four important generalizations about buffers. They act by neutralizing excess hydrogen ions, thereby maintaining the. A biological buffer is an. What Does A Buffer Do In Biology.
From www.youtube.com
pH and buffers overview for biology YouTube What Does A Buffer Do In Biology In this way, a biological buffer. A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. The most relevant systems for biology are the carbonic acid/carbonate buffering system, which controls blood ph and cells and. Four important generalizations about buffers. Biological buffers are organic substances that help regulate the ph level. What Does A Buffer Do In Biology.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Does A Buffer Do In Biology A buffer is composed of an equilibrium mixture of a weak acid (ha) and its conjugate weak base (a. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are important in biological systems because of their ability to maintain. Buffers are solutions that. What Does A Buffer Do In Biology.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID5687114 What Does A Buffer Do In Biology In this way, a biological buffer. A buffer is a solution containing either a weak acid and its salt or a weak base and its salt, which is resistant to changes in ph. They act by neutralizing excess hydrogen ions, thereby maintaining the. Buffers are important in biological systems because of their ability to maintain. Four important generalizations about buffers.. What Does A Buffer Do In Biology.