How To Find K Given Ka . It should help to look at the form of the constants: 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Determine the ka for formic acid. First, the ph is used to calculate the [h] at equilibrium. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. An ice table is set up in order to determine the. Calculate the value of k c for the reaction: Ka is the acid ionization constant, and kb is the base ionization constant. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. The concentration of h + and ch 3 coo. They can be likened to either the lewis or bronsted. List the known values and plan the problem. Balanced equilibrium equation, \(k\) at a given temperature, and equations of related reactions.
from pt.slideshare.net
Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. Calculate the value of k c for the reaction: Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. They can be likened to either the lewis or bronsted. First, the ph is used to calculate the [h] at equilibrium. 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. An ice table is set up in order to determine the. The concentration of h + and ch 3 coo. It should help to look at the form of the constants: Ka is the acid ionization constant, and kb is the base ionization constant.
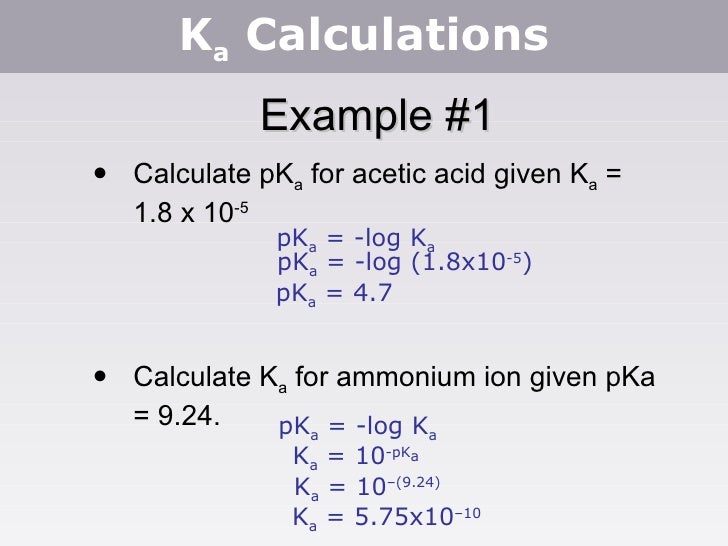
Tang 04 ka calculations 2
How To Find K Given Ka Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. An ice table is set up in order to determine the. Balanced equilibrium equation, \(k\) at a given temperature, and equations of related reactions. The concentration of h + and ch 3 coo. Calculate the value of k c for the reaction: Determine the ka for formic acid. Ka is the acid ionization constant, and kb is the base ionization constant. First, the ph is used to calculate the [h] at equilibrium. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. List the known values and plan the problem. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. They can be likened to either the lewis or bronsted. It should help to look at the form of the constants:
From www.youtube.com
pH of Weak Acids and Bases Percent Ionization Ka & Kb YouTube How To Find K Given Ka 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. They can be likened to either. How To Find K Given Ka.
From www.youtube.com
Calculating the Ka of an Unknown Weak Acid YouTube How To Find K Given Ka Calculate the value of k c for the reaction: Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. The concentration of h + and ch 3 coo. Determine the ka for formic acid. Balanced equilibrium. How To Find K Given Ka.
From www.youtube.com
Relationship between Ka and Kb for Conjugate AcidBase Pairs YouTube How To Find K Given Ka First, the ph is used to calculate the [h] at equilibrium. It should help to look at the form of the constants: List the known values and plan the problem. Determine the ka for formic acid. Balanced equilibrium equation, \(k\) at a given temperature, and equations of related reactions. An ice table is set up in order to determine the.. How To Find K Given Ka.
From www.youtube.com
Keq Equilibrium Constant (EVERYTHING YOU NEED TO KNOW CHEMISTRY) YouTube How To Find K Given Ka Balanced equilibrium equation, \(k\) at a given temperature, and equations of related reactions. The concentration of h + and ch 3 coo. Ka is the acid ionization constant, and kb is the base ionization constant. An ice table is set up in order to determine the. First, the ph is used to calculate the [h] at equilibrium. Determine the ka. How To Find K Given Ka.
From www.youtube.com
CHEM 201 Calculating Equilibrium Concentrations from K and Initial How To Find K Given Ka First, the ph is used to calculate the [h] at equilibrium. List the known values and plan the problem. They can be likened to either the lewis or bronsted. Determine the ka for formic acid. An ice table is set up in order to determine the. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +]. How To Find K Given Ka.
From www.youtube.com
Chemical Equilibrium Constant K Ice Tables Kp and Kc Membership How To Find K Given Ka It should help to look at the form of the constants: They can be likened to either the lewis or bronsted. An ice table is set up in order to determine the. Determine the ka for formic acid. List the known values and plan the problem. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations. How To Find K Given Ka.
From www.youtube.com
Calculating K from Concentration YouTube How To Find K Given Ka List the known values and plan the problem. They can be likened to either the lewis or bronsted. Calculate the value of k c for the reaction: First, the ph is used to calculate the [h] at equilibrium. The concentration of h + and ch 3 coo. Balanced equilibrium equation, \(k\) at a given temperature, and equations of related reactions.. How To Find K Given Ka.
From jackwestin.com
Equilibrium Constants Ka And Kb Pka Pkb Acid Base Equilibria MCAT How To Find K Given Ka First, the ph is used to calculate the [h] at equilibrium. Determine the ka for formic acid. 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. An ice table is set up in order to determine the. Ka is the acid ionization constant, and kb is the base ionization. How To Find K Given Ka.
From www.youtube.com
Using Keq to find concentrations YouTube How To Find K Given Ka First, the ph is used to calculate the [h] at equilibrium. List the known values and plan the problem. Calculate the value of k c for the reaction: The concentration of h + and ch 3 coo. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h. How To Find K Given Ka.
From www.youtube.com
How to Calculate pH from Ka in Weak Acids YouTube How To Find K Given Ka Calculate the value of k c for the reaction: Determine the ka for formic acid. The concentration of h + and ch 3 coo. 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. It should help to look at the form of the constants: An ice table is set. How To Find K Given Ka.
From www.youtube.com
Ka from pH and a concentration YouTube How To Find K Given Ka 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Determine the ka for formic acid. They can be likened to either the lewis or bronsted. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient. How To Find K Given Ka.
From www.youtube.com
How to Calculate Ka (Acid Dissociation Constant) of a Weak Acid How To Find K Given Ka Balanced equilibrium equation, \(k\) at a given temperature, and equations of related reactions. List the known values and plan the problem. An ice table is set up in order to determine the. Determine the ka for formic acid. Ka is the acid ionization constant, and kb is the base ionization constant. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n. How To Find K Given Ka.
From www.slideserve.com
PPT Determine Ka of a Weak Acid PowerPoint Presentation, free How To Find K Given Ka Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. An ice table is set up in order to determine the. The concentration of h + and ch 3 coo. Calculate the value of k c. How To Find K Given Ka.
From www.ck12.org
K[a], K[b], pK[a] , and pK[b] Example 1 ( Video ) Chemistry CK12 How To Find K Given Ka 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. An ice table is set up in order to determine the. Calculate. How To Find K Given Ka.
From www.youtube.com
Calculating Ka from ionization YouTube How To Find K Given Ka Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. Determine the ka for formic acid. Calculate the value of k c for the reaction: Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x. How To Find K Given Ka.
From www.youtube.com
Calculating Ka using an ICE table YouTube How To Find K Given Ka List the known values and plan the problem. It should help to look at the form of the constants: Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. An ice table is set up in. How To Find K Given Ka.
From haipernews.com
How To Calculate Kc Given Concentration Haiper How To Find K Given Ka List the known values and plan the problem. 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. First, the ph is used to calculate the [h] at equilibrium. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n]. How To Find K Given Ka.
From www.wikihow.com
How to Find Ka from pKa Plus pKa to Ka & 5 Sample Problems How To Find K Given Ka Balanced equilibrium equation, \(k\) at a given temperature, and equations of related reactions. Ka is the acid ionization constant, and kb is the base ionization constant. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn].. How To Find K Given Ka.
From www.youtube.com
Calculate Ka from Kb and vice versa YouTube How To Find K Given Ka List the known values and plan the problem. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. They can be likened to either the lewis or bronsted. An ice table is set up in order to determine the. It should help. How To Find K Given Ka.
From www.youtube.com
Ka and Kb values of conjugate acidbase pairs YouTube How To Find K Given Ka Ka is the acid ionization constant, and kb is the base ionization constant. First, the ph is used to calculate the [h] at equilibrium. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. 2 n. How To Find K Given Ka.
From ar.inspiredpencil.com
Ka Values And Acids How To Find K Given Ka The concentration of h + and ch 3 coo. Calculate the value of k c for the reaction: Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +]. How To Find K Given Ka.
From www.slideserve.com
PPT The Equilibrium Constant, K, and The Reaction Quotient, Q How To Find K Given Ka Ka is the acid ionization constant, and kb is the base ionization constant. 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. An ice table is set up in order to determine the. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n. How To Find K Given Ka.
From www.youtube.com
How To Determine The Units Of The Rate Constant K Chemical How To Find K Given Ka Ka is the acid ionization constant, and kb is the base ionization constant. An ice table is set up in order to determine the. List the known values and plan the problem. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h. How To Find K Given Ka.
From www.youtube.com
Using Ka to calculate pH YouTube How To Find K Given Ka An ice table is set up in order to determine the. Calculate the value of k c for the reaction: They can be likened to either the lewis or bronsted. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n,. How To Find K Given Ka.
From www.youtube.com
CHEMISTRY 201 Calculating Ka for a weak acid from pH YouTube How To Find K Given Ka They can be likened to either the lewis or bronsted. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. Calculate the value of k c for the reaction: Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x. How To Find K Given Ka.
From sciencenotes.org
pH, pKa, Ka, pKb, and Kb in Chemistry How To Find K Given Ka The concentration of h + and ch 3 coo. List the known values and plan the problem. An ice table is set up in order to determine the. Ka is the acid ionization constant, and kb is the base ionization constant. It should help to look at the form of the constants: They can be likened to either the lewis. How To Find K Given Ka.
From www.slideserve.com
PPT Ka, Kb PowerPoint Presentation, free download ID6823732 How To Find K Given Ka The concentration of h + and ch 3 coo. Determine the ka for formic acid. Calculate the value of k c for the reaction: Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the chemical equation. It should help to look at the form. How To Find K Given Ka.
From www.youtube.com
Given Two Angles and One Side AAS Ex 1 YouTube How To Find K Given Ka The concentration of h + and ch 3 coo. It should help to look at the form of the constants: Ka is the acid ionization constant, and kb is the base ionization constant. They can be likened to either the lewis or bronsted. 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using. How To Find K Given Ka.
From www.youtube.com
Calculating Ksp and Ka/Kb YouTube How To Find K Given Ka The concentration of h + and ch 3 coo. Determine the ka for formic acid. Ka is the acid ionization constant, and kb is the base ionization constant. An ice table is set up in order to determine the. They can be likened to either the lewis or bronsted. 2 n 2 o (g) + 3 o 2 (g) 2. How To Find K Given Ka.
From topptutors.blogspot.com
How To Find Ph From Molarity And Ka How To Find K Given Ka 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. An ice table is set up in order to determine the. They can be likened to either the lewis or bronsted. Ka is the acid ionization constant, and kb is the base ionization constant. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e. How To Find K Given Ka.
From www.youtube.com
Calculating K from DeltaG (Equilibrium Constant) YouTube How To Find K Given Ka Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants, each raised to its coefficient in the. How To Find K Given Ka.
From lessonbergininviting.z21.web.core.windows.net
How To Find K In Algebra How To Find K Given Ka List the known values and plan the problem. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka = [hx+][cnx−] [hcn]. The concentration of h + and ch 3 coo. It should help to look at the form of. How To Find K Given Ka.
From www.youtube.com
Calculating pH from Ka YouTube How To Find K Given Ka The concentration of h + and ch 3 coo. First, the ph is used to calculate the [h] at equilibrium. It should help to look at the form of the constants: An ice table is set up in order to determine the. Balanced equilibrium equation, \(k\) at a given temperature, and equations of related reactions. Find \(k\) by writing each. How To Find K Given Ka.
From www.youtube.com
How to convert between Ka and pKa (or Kb and pKb) YouTube How To Find K Given Ka 2 n 2 o (g) + 3 o 2 (g) 2 n 2 o 4 (g), using the following information. It should help to look at the form of the constants: Calculate the value of k c for the reaction: They can be likened to either the lewis or bronsted. An ice table is set up in order to determine. How To Find K Given Ka.
From pt.slideshare.net
Tang 04 ka calculations 2 How To Find K Given Ka Calculate the value of k c for the reaction: First, the ph is used to calculate the [h] at equilibrium. The concentration of h + and ch 3 coo. Keq = [nhx4x+][cnx−] [hcn][nhx3] k e q = [n h x 4 x +] [c n x −] [h c n] [n h x 3] for hcn h c n, ka. How To Find K Given Ka.