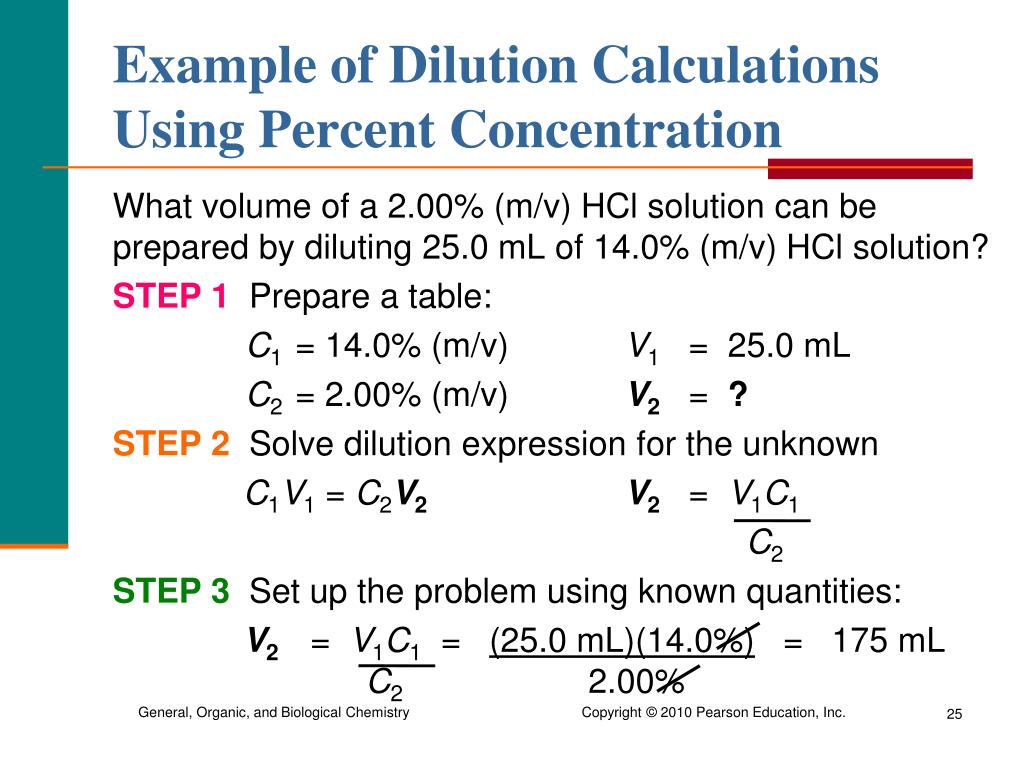
What Is The Dilution Equation . State whether the concentration of a solution is directly or indirectly proportional to its volume. C1 is the concentration of the starting solution. You can calculate the concentration of a solution following a dilution by applying this equation: Concentration is the removal of solvent, which increases the concentration of. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Wolfram|alpha computes dilutions using the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. V1 is the volume of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. This yields the most general form of the dilution equation:
from www.slideserve.com
This yields the most general form of the dilution equation: Wolfram|alpha computes dilutions using the. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. State whether the concentration of a solution is directly or indirectly proportional to its volume. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Concentration is the removal of solvent, which increases the concentration of. V1 is the volume of the starting solution.
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID
What Is The Dilution Equation Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C1 is the concentration of the starting solution. Wolfram|alpha computes dilutions using the. State whether the concentration of a solution is directly or indirectly proportional to its volume. This yields the most general form of the dilution equation: Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Concentration is the removal of solvent, which increases the concentration of. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. You can calculate the concentration of a solution following a dilution by applying this equation: V1 is the volume of the starting solution.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog What Is The Dilution Equation Wolfram|alpha computes dilutions using the. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. State whether the concentration of a solution is directly or indirectly proportional to its volume. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. This yields the most general. What Is The Dilution Equation.
From www.youtube.com
How to Calculate Dilution Factor YouTube What Is The Dilution Equation V1 is the volume of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C1 is the concentration of the starting solution. You can calculate the concentration of a solution following a dilution by applying this equation: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. What Is The Dilution Equation.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved What Is The Dilution Equation C1 is the concentration of the starting solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. State whether the. What Is The Dilution Equation.
From exopkbipv.blob.core.windows.net
Dilution Equation Example Chemistry at Wanda King blog What Is The Dilution Equation C1 is the concentration of the starting solution. V1 is the volume of the starting solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Dilution calculations allow one to determine how much solvent to add or what the final. What Is The Dilution Equation.
From www.youtube.com
Chem121 Dilution Equation 8 5 YouTube What Is The Dilution Equation State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. The formula for calculating a dilution is (c1) (v1) = (c2) (v2). What Is The Dilution Equation.
From www.youtube.com
Calculating Dilutions C1V1=C2V2 PowerPoint YouTube What Is The Dilution Equation State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. V1 is the volume of the starting solution. C1 is the concentration of the starting solution. You can calculate the concentration of a solution following a dilution by applying this equation: M i v. What Is The Dilution Equation.
From www.showme.com
Dilution calculation Science, Chemicalreactions, Biology ShowMe What Is The Dilution Equation M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. You can calculate the concentration of a solution following a dilution by applying this equation: V1 is the volume of the starting solution. This yields the most general form of the. What Is The Dilution Equation.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript What Is The Dilution Equation Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. This yields the most general form of the dilution equation: Wolfram|alpha computes dilutions using the. C1 is the concentration of the starting solution. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of. What Is The Dilution Equation.
From www.youtube.com
Stock Solutions & Dilutions YouTube What Is The Dilution Equation Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. You can calculate the concentration of a solution following a dilution by applying this equation: Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. V1 is the volume of the starting. What Is The Dilution Equation.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube What Is The Dilution Equation You can calculate the concentration of a solution following a dilution by applying this equation: \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. C1 is the concentration of the starting solution. The formula for calculating. What Is The Dilution Equation.
From www.scribd.com
Dilution (Equation) Solution Applied And Interdisciplinary Physics What Is The Dilution Equation You can calculate the concentration of a solution following a dilution by applying this equation: Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution. What Is The Dilution Equation.
From chem2u.blogspot.com
chem2U Dilution method formula What Is The Dilution Equation C1 is the concentration of the starting solution. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Dilution calculations allow one to determine. What Is The Dilution Equation.
From www.youtube.com
Chem143 Dilution Equation YouTube What Is The Dilution Equation V1 is the volume of the starting solution. C1 is the concentration of the starting solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. This yields the most general form of the dilution equation: Concentration is the removal of. What Is The Dilution Equation.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog What Is The Dilution Equation C1 is the concentration of the starting solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. Concentration. What Is The Dilution Equation.
From www.pdfprof.com
how to calculate dilution factor What Is The Dilution Equation \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. What Is The Dilution Equation.
From www.youtube.com
Quickvideo Using the dilution equation M1V1=M2V2 YouTube What Is The Dilution Equation Wolfram|alpha computes dilutions using the. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the. What Is The Dilution Equation.
From www.youtube.com
Dilution Problems, Chemistry, Molarity & Concentration Examples What Is The Dilution Equation M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. You can calculate the concentration of a solution following a dilution by applying this equation: Dilution calculations allow one to determine how much solvent to add or what the final concentration. What Is The Dilution Equation.
From www.medicine.mcgill.ca
Serial Dilutions What Is The Dilution Equation This yields the most general form of the dilution equation: C1 is the concentration of the starting solution. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. M i v i = m f v f where m is molarity, v is volume, and the subscripts i. What Is The Dilution Equation.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID What Is The Dilution Equation \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Concentration is the removal of solvent, which increases the concentration of. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution.. What Is The Dilution Equation.
From klafwpnrs.blob.core.windows.net
Dilution Formula Explanation at John Schell blog What Is The Dilution Equation M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Wolfram|alpha computes dilutions using the. Dilution calculations allow one to determine how much solvent to add or what. What Is The Dilution Equation.
From www.slideserve.com
PPT Molarity & Dilution PowerPoint Presentation, free download ID What Is The Dilution Equation Wolfram|alpha computes dilutions using the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. This yields the most general form of the dilution equation: The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. Dilution is the addition of solvent,. What Is The Dilution Equation.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free What Is The Dilution Equation Wolfram|alpha computes dilutions using the. You can calculate the concentration of a solution following a dilution by applying this equation: V1 is the volume of the starting solution. This yields the most general form of the dilution equation: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f. What Is The Dilution Equation.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) What Is The Dilution Equation Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. C1 is the concentration of the starting solution. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of. What Is The Dilution Equation.
From exopkbipv.blob.core.windows.net
Dilution Equation Example Chemistry at Wanda King blog What Is The Dilution Equation \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution calculations allow one to determine how much solvent to add. What Is The Dilution Equation.
From www.youtube.com
Dilution in biology lab How to use the equation C1V1 = C2V2 YouTube What Is The Dilution Equation State whether the concentration of a solution is directly or indirectly proportional to its volume. Wolfram|alpha computes dilutions using the. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. V1 is. What Is The Dilution Equation.
From www.youtube.com
Dilutions using equations YouTube What Is The Dilution Equation State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of. V1 is the volume of the starting solution. This yields the most general form of the dilution equation: Wolfram|alpha computes dilutions using the. The formula for calculating a dilution is (c1) (v1) = (c2). What Is The Dilution Equation.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution What Is The Dilution Equation Wolfram|alpha computes dilutions using the. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. You can calculate the concentration of a solution following a dilution by applying this equation: State whether the concentration of a solution. What Is The Dilution Equation.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID What Is The Dilution Equation M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Wolfram|alpha computes dilutions using the. C1 is the concentration of the starting solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. State whether. What Is The Dilution Equation.
From www.youtube.com
Using the Dilution Equation YouTube What Is The Dilution Equation State whether the concentration of a solution is directly or indirectly proportional to its volume. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and a subscript of 2 means after dilution. V1 is the volume of the starting solution. This yields the most. What Is The Dilution Equation.
From www.youtube.com
Dilution Equation 1 YouTube What Is The Dilution Equation V1 is the volume of the starting solution. Concentration is the removal of solvent, which increases the concentration of. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. You can calculate the concentration of a solution following a dilution by. What Is The Dilution Equation.
From www.youtube.com
How to Use the Dilution Equation YouTube What Is The Dilution Equation M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. Wolfram|alpha computes dilutions using the. C1 is the concentration of the starting solution. Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. You can. What Is The Dilution Equation.
From www.slideshare.net
Molarity and dilution What Is The Dilution Equation Wolfram|alpha computes dilutions using the. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and final values. \[c_1v_1=c_2v_2\] to summarize, c stands for the concentration of the solution, v for the volume of the solution, a subscript of 1 means before dilution, and. What Is The Dilution Equation.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and What Is The Dilution Equation Dilution is the addition of solvent, which decreases the concentration of the solute in the solution. Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. Wolfram|alpha computes dilutions using the. C1 is the concentration of the starting solution. M i v i = m f v f. What Is The Dilution Equation.
From loegnwkyw.blob.core.windows.net
Serial Dilution Equation Examples at Keith Rush blog What Is The Dilution Equation Dilution calculations allow one to determine how much solvent to add or what the final concentration is after the addition of solvent. Concentration is the removal of solvent, which increases the concentration of. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer to the initial and. What Is The Dilution Equation.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube What Is The Dilution Equation The formula for calculating a dilution is (c1) (v1) = (c2) (v2) where. C1 is the concentration of the starting solution. V1 is the volume of the starting solution. Wolfram|alpha computes dilutions using the. State whether the concentration of a solution is directly or indirectly proportional to its volume. Concentration is the removal of solvent, which increases the concentration of.. What Is The Dilution Equation.