Titration Weak Acid . The midpoint of a titration is defined as the point at. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. Initially, the ph of a weak. there are two basic types of acid base titrations, indicator and potentiometric. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. titration of a weak acid with a strong base. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. one point in the titration of a weak acid or a weak base is particularly important: The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3.
from chemistnotes.com
there are two basic types of acid base titrations, indicator and potentiometric. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. one point in the titration of a weak acid or a weak base is particularly important: titration of a weak acid with a strong base. Initially, the ph of a weak. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. The midpoint of a titration is defined as the point at. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic.
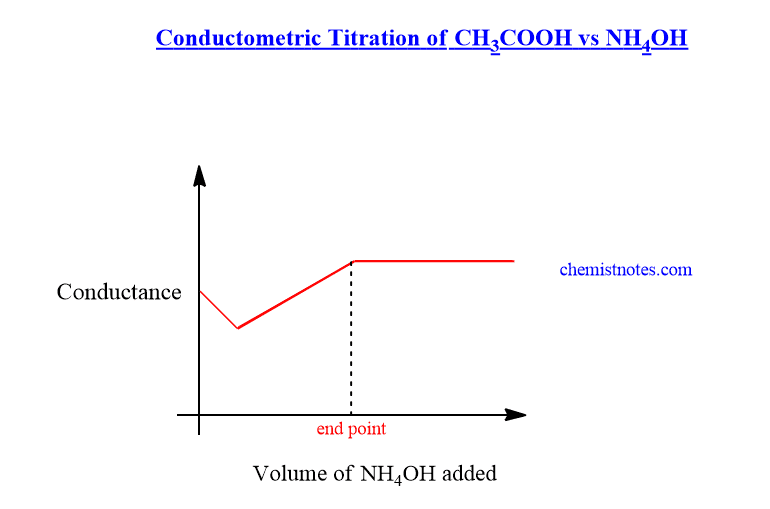
Conductometric titration, easy principle, curves, 3 advantages
Titration Weak Acid The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. there are two basic types of acid base titrations, indicator and potentiometric. one point in the titration of a weak acid or a weak base is particularly important: ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. Initially, the ph of a weak. The midpoint of a titration is defined as the point at. titration of a weak acid with a strong base. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Weak Acid ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. Initially, the ph of a weak. one point in the titration of a weak acid or a weak. Titration Weak Acid.
From www.youtube.com
Weak Acid with Strong Base Titration 1 YouTube Titration Weak Acid The midpoint of a titration is defined as the point at. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. there are two basic types of acid. Titration Weak Acid.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Titration Weak Acid there are two basic types of acid base titrations, indicator and potentiometric. Initially, the ph of a weak. titration of a weak acid with a strong base. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. The titration curve shown in figure 1 is for. Titration Weak Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Weak Acid there are two basic types of acid base titrations, indicator and potentiometric. The midpoint of a titration is defined as the point at. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. titration of a weak acid with a strong base. one point in. Titration Weak Acid.
From www.chegg.com
Solved CHEMISTRY. IDENTIFY WEAK ACID USING TITRATION CURVE A Titration Weak Acid there are two basic types of acid base titrations, indicator and potentiometric. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. one point in the titration of a weak acid or a weak base is particularly important: titration of a weak acid with a. Titration Weak Acid.
From www.youtube.com
17.3 Weak Acid Strong Base Titration Curve YouTube Titration Weak Acid titration of a weak acid with a strong base. one point in the titration of a weak acid or a weak base is particularly important: The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. The midpoint of a titration is defined as the point at. ph indicators. Titration Weak Acid.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Weak Acid titration of a weak acid with a strong base. there are two basic types of acid base titrations, indicator and potentiometric. The midpoint of a titration is defined as the point at. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. one point in. Titration Weak Acid.
From www.youtube.com
Weak basestrong acid titrations Acids and bases AP Chemistry Titration Weak Acid one point in the titration of a weak acid or a weak base is particularly important: in titrating a weak acid with a strong base, different calculation methods are applied at various stages. there are two basic types of acid base titrations, indicator and potentiometric. the titration of a weak acid with a strong base involves. Titration Weak Acid.
From www.slideserve.com
PPT Titrations of Weak Acids and Weak Bases PowerPoint Presentation Titration Weak Acid titration of a weak acid with a strong base. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. the titration of a weak acid with a. Titration Weak Acid.
From www.youtube.com
titration weak acid part 1 YouTube Titration Weak Acid The midpoint of a titration is defined as the point at. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. titration of a weak acid with a strong base. there are two basic types of acid base titrations, indicator and potentiometric. Initially, the ph of. Titration Weak Acid.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Titration Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. there are two basic types of acid base titrations, indicator and potentiometric. titration of a weak acid with a strong base. one point in the titration of a weak acid or a weak base is. Titration Weak Acid.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration Weak Acid in titrating a weak acid with a strong base, different calculation methods are applied at various stages. Initially, the ph of a weak. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. the titration of a weak acid with a strong base involves the direct. Titration Weak Acid.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic Titration Weak Acid there are two basic types of acid base titrations, indicator and potentiometric. The midpoint of a titration is defined as the point at. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. Initially, the ph of a weak. The titration curve shown in figure 1 is. Titration Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. there are two basic types of acid base titrations, indicator and potentiometric. one point in the titration of a weak acid or a weak base is particularly important: titration of a weak acid with a. Titration Weak Acid.
From psu.pb.unizin.org
14.7 AcidBase Titrations Chemistry 112 Chapters 1217 of OpenStax Titration Weak Acid Initially, the ph of a weak. The midpoint of a titration is defined as the point at. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. ph. Titration Weak Acid.
From www.youtube.com
Conductometric titration of weak acid and weak base (weak acid vs weak Titration Weak Acid one point in the titration of a weak acid or a weak base is particularly important: The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. there are two basic types of acid base titrations, indicator and potentiometric. titration of a weak acid with a strong base. Initially,. Titration Weak Acid.
From wisc.pb.unizin.org
M16Q6 Titration of a Weak Acid with a Strong Base; Titration of a Weak Titration Weak Acid ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. there are two basic types of acid base titrations, indicator and potentiometric. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. in titrating. Titration Weak Acid.
From exosxgjvz.blob.core.windows.net
Weak Acid Titration Curve Buffer Region at Paula Rivera blog Titration Weak Acid titration of a weak acid with a strong base. there are two basic types of acid base titrations, indicator and potentiometric. The midpoint of a titration is defined as the point at. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. ph indicators are. Titration Weak Acid.
From studylib.net
Titration Curve weak base with strong acid START Titration Weak Acid titration of a weak acid with a strong base. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. The titration curve shown in figure. Titration Weak Acid.
From present5.com
AcidBase Titrations Barb Fallon AP Chemistry June 2007 Titration Weak Acid ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. Initially, the ph of a weak. there are two basic types of acid base titrations, indicator and potentiometric. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3.. Titration Weak Acid.
From slideplayer.com
Weak Acid Strong Base Titration ppt download Titration Weak Acid one point in the titration of a weak acid or a weak base is particularly important: The midpoint of a titration is defined as the point at. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. the titration of a weak acid with a strong base involves the. Titration Weak Acid.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID2134619 Titration Weak Acid one point in the titration of a weak acid or a weak base is particularly important: there are two basic types of acid base titrations, indicator and potentiometric. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. The titration curve shown in figure 1 is for the titration of. Titration Weak Acid.
From www.slideserve.com
PPT Titrations of Weak Acids and Weak Bases PowerPoint Presentation Titration Weak Acid The midpoint of a titration is defined as the point at. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. one point in the titration of a weak acid or a weak base is particularly important: in titrating a weak acid with a strong base, different calculation methods. Titration Weak Acid.
From www.writework.com
Titration of amino acids WriteWork Titration Weak Acid Initially, the ph of a weak. there are two basic types of acid base titrations, indicator and potentiometric. The midpoint of a titration is defined as the point at. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. one point in the titration of a. Titration Weak Acid.
From dxozbvbve.blob.core.windows.net
Titration Weak Acid Weak Base at Minnie Cortez blog Titration Weak Acid titration of a weak acid with a strong base. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. one point in the titration of a weak acid or a weak base is particularly important: the titration of a weak acid with a strong base involves the direct transfer. Titration Weak Acid.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid Titration Weak Acid The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. titration of a weak acid with a strong base. there are two basic types of acid base titrations, indicator and potentiometric. Initially, the ph of a weak. the titration of a weak acid with a strong base involves. Titration Weak Acid.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Titration Weak Acid ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. Initially, the ph of a weak. titration of a weak acid with a strong base. one point in. Titration Weak Acid.
From webmis.highland.cc.il.us
AcidBase Titrations Titration Weak Acid Initially, the ph of a weak. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. titration of a weak acid with a strong base. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. The midpoint of a titration is defined. Titration Weak Acid.
From www.youtube.com
Titration weak acid calculations 2017 YouTube Titration Weak Acid Initially, the ph of a weak. The midpoint of a titration is defined as the point at. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. one point in the titration. Titration Weak Acid.
From courses.lumenlearning.com
AcidBase Titrations Chemistry for Majors Titration Weak Acid one point in the titration of a weak acid or a weak base is particularly important: The midpoint of a titration is defined as the point at. in titrating a weak acid with a strong base, different calculation methods are applied at various stages. there are two basic types of acid base titrations, indicator and potentiometric. Initially,. Titration Weak Acid.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Weak Acid one point in the titration of a weak acid or a weak base is particularly important: ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. The midpoint of a titration is defined as the point at. The titration curve shown in figure 1 is for the. Titration Weak Acid.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID Titration Weak Acid the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. there are two basic types of acid base titrations, indicator and potentiometric. one point. Titration Weak Acid.
From chemistnotes.com
Conductometric titration, easy principle, curves, 3 advantages Titration Weak Acid in titrating a weak acid with a strong base, different calculation methods are applied at various stages. The titration curve shown in figure 1 is for the titration of 25.00 ml of 0.100 m ch 3. Initially, the ph of a weak. one point in the titration of a weak acid or a weak base is particularly important:. Titration Weak Acid.
From mungfali.com
Weak Acid Vs Strong Base Titration Curve Titration Weak Acid in titrating a weak acid with a strong base, different calculation methods are applied at various stages. ph indicators are weak acids or weak bases that have different colors based on different chemical structures in acidic or basic. Initially, the ph of a weak. titration of a weak acid with a strong base. The midpoint of a. Titration Weak Acid.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Titration Weak Acid The midpoint of a titration is defined as the point at. titration of a weak acid with a strong base. one point in the titration of a weak acid or a weak base is particularly important: there are two basic types of acid base titrations, indicator and potentiometric. ph indicators are weak acids or weak bases. Titration Weak Acid.