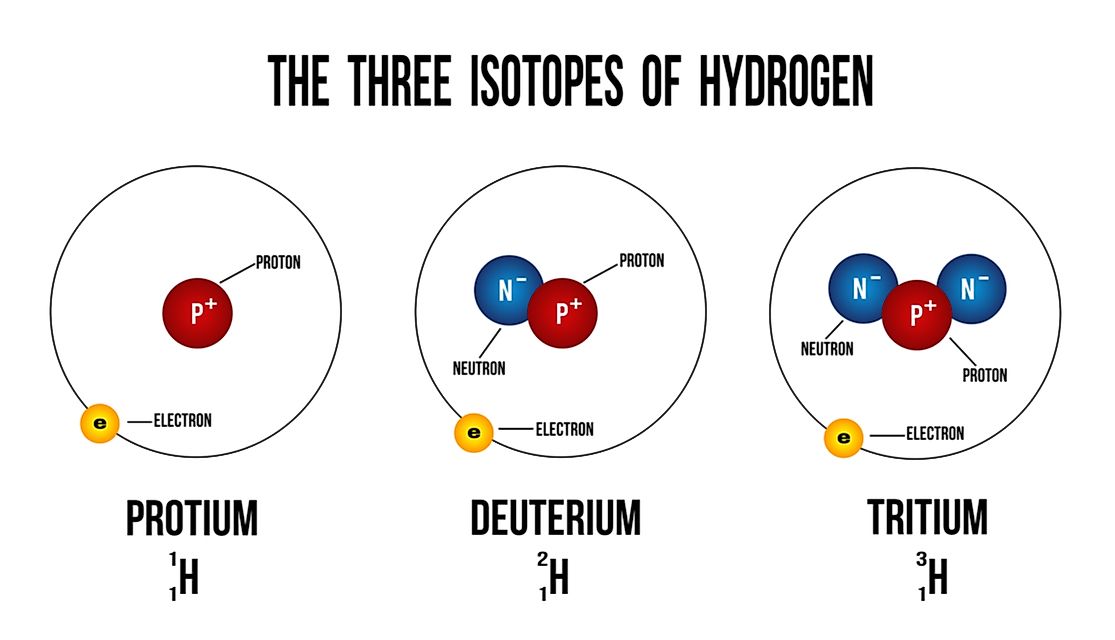
Which Chlorine Isotope Is More Abundant . 35 cl and 37 cl with. There are only two stable isotopes: In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. An ion with two chlorine atoms has three possible isotope combinations. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. There are two stable isotopes, 35 cl. 【solved】click here to get an answer to your question : This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring chlorine. If more than one chlorine atom is present, the isotope abundance is more complex. We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The individual nuclides differ in the number of. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in 24 isotopes and some nuclear isomers of the chemical element chlorine are known.
from www.worldatlas.com
24 isotopes and some nuclear isomers of the chemical element chlorine are known. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. There are two stable isotopes, 35 cl. 【solved】click here to get an answer to your question : There are only two stable isotopes: This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. The individual nuclides differ in the number of. An ion with two chlorine atoms has three possible isotope combinations.
What Is an Isotope? WorldAtlas
Which Chlorine Isotope Is More Abundant 【solved】click here to get an answer to your question : In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. An ion with two chlorine atoms has three possible isotope combinations. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring chlorine. There are two stable isotopes, 35 cl. We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 【solved】click here to get an answer to your question : 35 cl and 37 cl with. If more than one chlorine atom is present, the isotope abundance is more complex. There are only two stable isotopes: 24 isotopes and some nuclear isomers of the chemical element chlorine are known. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in The individual nuclides differ in the number of.
From www.youtube.com
Atomic Mass How to Calculate Isotope Abundance YouTube Which Chlorine Isotope Is More Abundant 24 isotopes and some nuclear isomers of the chemical element chlorine are known. We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass.. Which Chlorine Isotope Is More Abundant.
From www.worldatlas.com
What Is an Isotope? WorldAtlas Which Chlorine Isotope Is More Abundant This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in If more than one chlorine atom is present, the isotope abundance is more complex. 35 cl and 37 cl with. The individual nuclides differ in the number of. 【solved】click here to get an answer to your question : This is the most abundant isotope of. Which Chlorine Isotope Is More Abundant.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6461 Science Which Chlorine Isotope Is More Abundant 24 isotopes and some nuclear isomers of the chemical element chlorine are known. If more than one chlorine atom is present, the isotope abundance is more complex. The individual nuclides differ in the number of. 35 cl and 37 cl with. 【solved】click here to get an answer to your question : There are two stable isotopes, 35 cl. In the. Which Chlorine Isotope Is More Abundant.
From www.chegg.com
Solved 9) Rubidium, lithium, nitrogen and neon each have two Which Chlorine Isotope Is More Abundant The individual nuclides differ in the number of. 【solved】click here to get an answer to your question : There are only two stable isotopes: An ion with two chlorine atoms has three possible isotope combinations. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. In the. Which Chlorine Isotope Is More Abundant.
From www.numerade.com
SOLVED [Choose ] Negatively charged ion of Carbon Isotope of Chlorine Which Chlorine Isotope Is More Abundant In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. 【solved】click here to get an answer to your question : There are only two stable isotopes: If more than one chlorine atom is present, the isotope abundance is more complex. 53 rows chlorine (17 cl) has 25 isotopes, ranging. Which Chlorine Isotope Is More Abundant.
From slideplayer.com
Average Atomic Mass In nature, most elements are a mixture of different Which Chlorine Isotope Is More Abundant This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring chlorine. The individual nuclides differ in the number of. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 24 isotopes and some nuclear isomers of the chemical element chlorine are known.. Which Chlorine Isotope Is More Abundant.
From studiousguy.com
Chlorine (Cl) Properties & Uses StudiousGuy Which Chlorine Isotope Is More Abundant There are only two stable isotopes: We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring. Which Chlorine Isotope Is More Abundant.
From slideplayer.com
Atoms All matter is made of them ppt download Which Chlorine Isotope Is More Abundant 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 【solved】click here to get an answer to your question : If more than one chlorine atom is present, the isotope abundance is more complex. This is the most abundant isotope of chlorine, making up about 75.78% of. Which Chlorine Isotope Is More Abundant.
From slideplayer.com
What Is in an Atom? What is the difference between protons, neutrons Which Chlorine Isotope Is More Abundant In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. An ion with two chlorine atoms has three possible isotope combinations. There are two stable isotopes, 35 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and. Which Chlorine Isotope Is More Abundant.
From martlabpro.com
What Is The Difference Between A Stable And Unstable Isotope? MartLabPro Which Chlorine Isotope Is More Abundant 24 isotopes and some nuclear isomers of the chemical element chlorine are known. We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in 53 rows chlorine (17 cl) has. Which Chlorine Isotope Is More Abundant.
From www.slideserve.com
PPT Average Atomic Mass PowerPoint Presentation, free download ID Which Chlorine Isotope Is More Abundant There are only two stable isotopes: An ion with two chlorine atoms has three possible isotope combinations. We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. 【solved】click here to get. Which Chlorine Isotope Is More Abundant.
From yoo.rs
What is the isotope? Yoors Which Chlorine Isotope Is More Abundant An ion with two chlorine atoms has three possible isotope combinations. 35 cl and 37 cl with. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 【solved】click here to get an answer to. Which Chlorine Isotope Is More Abundant.
From brainly.com
Chlorine has two isotopes, 35Cl and 37Cl; 75.77 of chlorine is 35Cl Which Chlorine Isotope Is More Abundant The individual nuclides differ in the number of. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. 35 cl and 37 cl with. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in 24 isotopes and some nuclear isomers of the chemical element. Which Chlorine Isotope Is More Abundant.
From www.tpsearchtool.com
What Are Isotopes Definition Types Examples Images Which Chlorine Isotope Is More Abundant There are only two stable isotopes: We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. An ion with two chlorine atoms has three possible isotope combinations. The individual nuclides differ in the number of. There are two stable isotopes, 35 cl. 24 isotopes. Which Chlorine Isotope Is More Abundant.
From byjus.com
40. Atomic weight of chlorine is 35.5.It has two isotopes of atomic Which Chlorine Isotope Is More Abundant An ion with two chlorine atoms has three possible isotope combinations. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring. Which Chlorine Isotope Is More Abundant.
From www.slideshare.net
Isotopes Which Chlorine Isotope Is More Abundant There are two stable isotopes, 35 cl. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring chlorine. 【solved】click here to get an answer to your question : 35 cl and 37 cl with. We will expect that the percent. Which Chlorine Isotope Is More Abundant.
From chiangmaiplaces.net
How Do You Know Which Isotope Is More Abundant? Quick Answer Which Chlorine Isotope Is More Abundant There are two stable isotopes, 35 cl. There are only two stable isotopes: The individual nuclides differ in the number of. 35 cl and 37 cl with. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. An ion with two chlorine atoms has three possible isotope combinations. 24 isotopes and some nuclear isomers of the. Which Chlorine Isotope Is More Abundant.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Which Chlorine Isotope Is More Abundant 【solved】click here to get an answer to your question : An ion with two chlorine atoms has three possible isotope combinations. There are only two stable isotopes: 24 isotopes and some nuclear isomers of the chemical element chlorine are known. 35 cl and 37 cl with. In the above, the most intense ion is set to 100% since this corresponds. Which Chlorine Isotope Is More Abundant.
From greekchlist.weebly.com
Chlorine atomic mass greekchlist Which Chlorine Isotope Is More Abundant An ion with two chlorine atoms has three possible isotope combinations. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in. Which Chlorine Isotope Is More Abundant.
From ar.inspiredpencil.com
Isotope Definition Which Chlorine Isotope Is More Abundant This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring chlorine. We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. An ion with two chlorine atoms has three possible isotope combinations. Chlorine has 24 isotopes with mass numbers. Which Chlorine Isotope Is More Abundant.
From dxorlvkyp.blob.core.windows.net
Chlorine Isotopes Notation at Roy Laster blog Which Chlorine Isotope Is More Abundant There are two stable isotopes, 35 cl. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers,. Which Chlorine Isotope Is More Abundant.
From slideplayer.com
Ch 6 Chemical Composition ppt download Which Chlorine Isotope Is More Abundant An ion with two chlorine atoms has three possible isotope combinations. The individual nuclides differ in the number of. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. 【solved】click here to get an answer to your question : 53 rows chlorine (17 cl) has 25 isotopes, ranging from. Which Chlorine Isotope Is More Abundant.
From slideplayer.com
Average Atomic Mass In nature, most elements are a mixture of different Which Chlorine Isotope Is More Abundant If more than one chlorine atom is present, the isotope abundance is more complex. 【solved】click here to get an answer to your question : There are only two stable isotopes: 24 isotopes and some nuclear isomers of the chemical element chlorine are known. 35 cl and 37 cl with. There are two stable isotopes, 35 cl. An ion with two. Which Chlorine Isotope Is More Abundant.
From www.numerade.com
SOLVED Nitrogen has two naturally occurring isotopes nitrogen14 and Which Chlorine Isotope Is More Abundant An ion with two chlorine atoms has three possible isotope combinations. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 35 cl and 37 cl with. There are only two stable isotopes: 【solved】click here to get an answer to your question : The individual nuclides differ. Which Chlorine Isotope Is More Abundant.
From www.chegg.com
Solved Which isotope in Zirconium is the least abundant? A B Which Chlorine Isotope Is More Abundant 35 cl and 37 cl with. There are two stable isotopes, 35 cl. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. There are only two stable isotopes: If more than one chlorine atom. Which Chlorine Isotope Is More Abundant.
From www.numerade.com
SOLVEDBoron, lithium, and nitrogen each have two stable isotopes. Use Which Chlorine Isotope Is More Abundant An ion with two chlorine atoms has three possible isotope combinations. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. We will expect that the percent abundance of the isotopes of an element. Which Chlorine Isotope Is More Abundant.
From www.chegg.com
Solved Which isotope is not Which Chlorine Isotope Is More Abundant We will expect that the percent abundance of the isotopes of an element is not the same which means that one isotope will be more abundant. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring chlorine. This pattern is apparent in. Which Chlorine Isotope Is More Abundant.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Which Chlorine Isotope Is More Abundant There are two stable isotopes, 35 cl. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in The individual nuclides differ in the number of. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 【solved】click here to get an answer to your question : In the above, the most intense. Which Chlorine Isotope Is More Abundant.
From www.youtube.com
Chlorine has two isotopes Cl35 and Cl37. Ratio present in nature is 31 Which Chlorine Isotope Is More Abundant This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in 24 isotopes and some nuclear isomers of the chemical element chlorine are known. This is the most abundant isotope of chlorine, making up about 75.78% of naturally occurring chlorine. If more than one chlorine atom is present, the isotope abundance is more complex. The individual. Which Chlorine Isotope Is More Abundant.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Which Chlorine Isotope Is More Abundant Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. An ion with two chlorine atoms has three possible isotope combinations. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in There are only two stable isotopes: We. Which Chlorine Isotope Is More Abundant.
From www.youtube.com
Among the two isotopes of chlorine are 3517cl and 3717cl. Which isotope Which Chlorine Isotope Is More Abundant There are two stable isotopes, 35 cl. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. The individual nuclides differ in the number of. An ion with two chlorine atoms has. Which Chlorine Isotope Is More Abundant.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram Which Chlorine Isotope Is More Abundant 【solved】click here to get an answer to your question : 35 cl and 37 cl with. This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in There are only two stable isotopes: If more than one chlorine atom is present, the isotope abundance is more complex. There are two stable isotopes, 35 cl. An ion. Which Chlorine Isotope Is More Abundant.
From www.coursehero.com
[Solved] Chlorine has two naturally occurring isotopes Chlorine 35 Which Chlorine Isotope Is More Abundant If more than one chlorine atom is present, the isotope abundance is more complex. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. 【solved】click here to get an answer to your question : In the above, the most intense ion is set. Which Chlorine Isotope Is More Abundant.
From www.slideserve.com
PPT 1. Atomic Structure PowerPoint Presentation, free download ID Which Chlorine Isotope Is More Abundant Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 53 rows chlorine (17 cl) has 25 isotopes, ranging from 28 cl to 52 cl, and two isomers, 34m cl and 38m cl. 24 isotopes and some nuclear isomers of the chemical element chlorine are known. There are two stable isotopes, 35 cl. In the above,. Which Chlorine Isotope Is More Abundant.
From www.expii.com
Isotope Notation — Overview & Examples Expii Which Chlorine Isotope Is More Abundant This pattern is apparent in the mass spectrum of ch 2 cl 2 shown in 24 isotopes and some nuclear isomers of the chemical element chlorine are known. The individual nuclides differ in the number of. 【solved】click here to get an answer to your question : An ion with two chlorine atoms has three possible isotope combinations. This is the. Which Chlorine Isotope Is More Abundant.