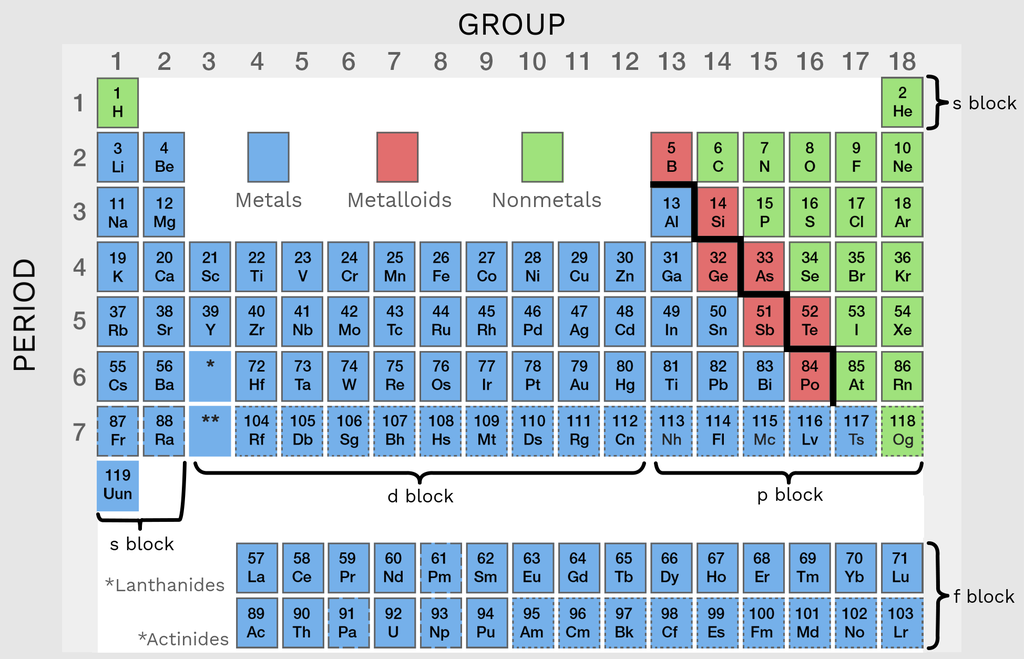
Metalloids Are In What Group . Boron, silicon, germanium, arsenic, antimony, and tellurium. Learn about the location, list, and characteristics of metalloids on the periodic table. metalloids can also be called semimetals. metalloids are elements that have properties intermediate between metals and nonmetals. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. They are brittle, poor conductors, and form amphoteric oxides. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. learn how to classify elements into three groups based on their properties and reactions. learn about the six metalloids in the periodic table: learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. Metals are shiny, conduct heat and electricity, and.
from theory.labster.com
They are brittle, poor conductors, and form amphoteric oxides. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Boron, silicon, germanium, arsenic, antimony, and tellurium. learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. metalloids can also be called semimetals. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. metalloids are elements that have properties intermediate between metals and nonmetals. Learn about the location, list, and characteristics of metalloids on the periodic table. Metals are shiny, conduct heat and electricity, and.
The periodic table Labster
Metalloids Are In What Group Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. metalloids can also be called semimetals. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. Learn about the location, list, and characteristics of metalloids on the periodic table. learn about the six metalloids in the periodic table: Boron, silicon, germanium, arsenic, antimony, and tellurium. metalloids are elements that have properties intermediate between metals and nonmetals. They are brittle, poor conductors, and form amphoteric oxides. learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. Metals are shiny, conduct heat and electricity, and. learn how to classify elements into three groups based on their properties and reactions. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,.
From byjus.com
Name all the metalloids Metalloids Are In What Group learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. learn how to classify elements into three groups based on their properties and reactions. They are brittle, poor conductors, and form amphoteric oxides. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,.. Metalloids Are In What Group.
From lookfordiagnosis.com
Metalloids Metalloids Are In What Group They are brittle, poor conductors, and form amphoteric oxides. metalloids are elements that have properties intermediate between metals and nonmetals. learn how to classify elements into three groups based on their properties and reactions. Learn about the location, list, and characteristics of metalloids on the periodic table. Metals are shiny, conduct heat and electricity, and. Find out how. Metalloids Are In What Group.
From ck12.org
Groups with Metalloids CK12 Foundation Metalloids Are In What Group learn how to classify elements into three groups based on their properties and reactions. learn about the six metalloids in the periodic table: Metals are shiny, conduct heat and electricity, and. They are brittle, poor conductors, and form amphoteric oxides. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. learn about. Metalloids Are In What Group.
From www.geeksforgeeks.org
Metalloids Definition, Position in Periodic Table, & Properties Metalloids Are In What Group learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. Learn about the location, list, and characteristics of metalloids on the periodic table. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. metalloids are elements that have properties intermediate between metals and nonmetals. Boron, silicon, germanium,. Metalloids Are In What Group.
From pediabay.com
Metalloids of the Periodic Table Pediabay Metalloids Are In What Group Metals are shiny, conduct heat and electricity, and. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. metalloids can also be called semimetals. Find out how they form covalent crystals, exhibit intermediate electronegativity, and. Metalloids Are In What Group.
From www.breakingatom.com
Metalloid Definition Metalloids Are In What Group Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. learn about the six metalloids in the periodic table: They are brittle, poor conductors, and form amphoteric oxides. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. metalloids can also be called semimetals.. Metalloids Are In What Group.
From newtondesk.com
Metalloids (Periodic Table) Properties, Uses, & Facts Metalloids Are In What Group the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. metalloids can also be called semimetals. Learn about. Metalloids Are In What Group.
From www.slideserve.com
PPT Chapter Eight PowerPoint Presentation, free download ID1801337 Metalloids Are In What Group Boron, silicon, germanium, arsenic, antimony, and tellurium. learn about the six metalloids in the periodic table: the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. metalloids are elements that have properties intermediate between metals and nonmetals. metalloids can also be called semimetals. Metals are shiny, conduct heat. Metalloids Are In What Group.
From sciencenotes.org
Metalloids Science Notes and Projects Metalloids Are In What Group learn about the six metalloids in the periodic table: Boron, silicon, germanium, arsenic, antimony, and tellurium. Learn about the location, list, and characteristics of metalloids on the periodic table. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. metalloids are elements that have properties intermediate between metals and nonmetals. learn about. Metalloids Are In What Group.
From sciencenotes.org
5 Examples of Metals, Metalloids, and Nonmetals Metalloids Are In What Group learn how to classify elements into three groups based on their properties and reactions. metalloids can also be called semimetals. Learn about the location, list, and characteristics of metalloids on the periodic table. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. metalloids are elements that have. Metalloids Are In What Group.
From byjus.com
In which block of the modern periodic table the metals, non metals and Metalloids Are In What Group Metals are shiny, conduct heat and electricity, and. Learn about the location, list, and characteristics of metalloids on the periodic table. Boron, silicon, germanium, arsenic, antimony, and tellurium. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications.. Metalloids Are In What Group.
From www.slideserve.com
PPT Metals, Metalloids, and Nonmetals PowerPoint Presentation, free Metalloids Are In What Group Boron, silicon, germanium, arsenic, antimony, and tellurium. Learn about the location, list, and characteristics of metalloids on the periodic table. learn about the six metalloids in the periodic table: learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. the term is normally applied to a group of between six and. Metalloids Are In What Group.
From knordslearning.com
Metalloids Periodic Table (With Images) Metalloids Are In What Group the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Metals are shiny, conduct heat and electricity, and. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. . Metalloids Are In What Group.
From www.differencebetween.com
Difference Between Metals and Metalloids Compare the Difference Metalloids Are In What Group metalloids can also be called semimetals. learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. They are brittle, poor conductors, and form amphoteric oxides. Learn about the location, list, and characteristics of metalloids on the periodic. Metalloids Are In What Group.
From www.adda247.com
What are metalloids? Definition, Properties and Example Metalloids Are In What Group Learn about the location, list, and characteristics of metalloids on the periodic table. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. Metals are shiny, conduct heat and electricity, and. They are brittle, poor conductors, and form amphoteric oxides. Boron, silicon, germanium, arsenic, antimony, and tellurium. learn about the seven metalloid. Metalloids Are In What Group.
From courses.lumenlearning.com
Periodicity Chemistry Metalloids Are In What Group the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. They are brittle, poor conductors, and form amphoteric oxides. metalloids can also be called semimetals. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. learn about the seven metalloid elements and their properties,. Metalloids Are In What Group.
From www.slideserve.com
PPT The Periodic Table of the Elements PowerPoint Presentation, free Metalloids Are In What Group Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. learn how to classify elements into three groups based on their properties and reactions. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. learn about the seven metalloid elements and their properties, uses,. Metalloids Are In What Group.
From www.haikudeck.com
Metalloids by Victoria Woodard Metalloids Are In What Group learn how to classify elements into three groups based on their properties and reactions. learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. They are brittle, poor conductors, and form amphoteric oxides. Learn about the location, list, and characteristics of metalloids on the periodic table. Metalloids are elements with intermediate characteristics. Metalloids Are In What Group.
From periodictableguide.com
Periodic table labeled with Metals Nonmetals and Metalloids Metalloids Are In What Group the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Learn about the location, list, and characteristics of metalloids on the periodic table. metalloids are elements that have properties intermediate between metals and nonmetals. They are brittle, poor conductors, and form amphoteric oxides. learn about the seven metalloid elements. Metalloids Are In What Group.
From www.nagwa.com
Question Video Identifying a Pair of Group 15 Elements That Are Metalloids Are In What Group Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. learn about the six metalloids in the periodic table: the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. metalloids can also be called semimetals. Learn about the location, list, and characteristics of metalloids. Metalloids Are In What Group.
From www.chemistrylearner.com
Metalloids Chemistry Learner Metalloids Are In What Group Boron, silicon, germanium, arsenic, antimony, and tellurium. They are brittle, poor conductors, and form amphoteric oxides. learn how to classify elements into three groups based on their properties and reactions. metalloids are elements that have properties intermediate between metals and nonmetals. the term is normally applied to a group of between six and nine elements (boron, silicon,. Metalloids Are In What Group.
From sciencenotes.org
List of Metalloids or Semimetals Metalloids Are In What Group metalloids can also be called semimetals. metalloids are elements that have properties intermediate between metals and nonmetals. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. Metals are shiny, conduct heat and electricity, and. They. Metalloids Are In What Group.
From ar.inspiredpencil.com
Periodic Table With Metals Metalloids And Nonmetals Labeled Metalloids Are In What Group learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. metalloids can also be called semimetals. learn how to classify elements into three groups based on their properties and reactions. Learn about the location, list, and characteristics of metalloids on the periodic table. metalloids are elements that have properties intermediate. Metalloids Are In What Group.
From knordslearning.com
Periodic Table Metals, Nonmetals & Metalloids (With Images) Metalloids Are In What Group learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. metalloids can also be called semimetals. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. Metals are shiny, conduct heat and electricity, and. Metalloids are elements with intermediate characteristics of metals and nonmetals, such. Metalloids Are In What Group.
From theory.labster.com
The periodic table Labster Metalloids Are In What Group learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. metalloids are elements that have properties intermediate between metals and nonmetals. Learn about the location, list, and characteristics of metalloids on the periodic table. Boron, silicon, germanium, arsenic, antimony, and tellurium. They are brittle, poor conductors, and form amphoteric oxides. learn. Metalloids Are In What Group.
From utedzz.blogspot.com
Periodic Table Metalloids Periodic Table Timeline Metalloids Are In What Group Learn about the location, list, and characteristics of metalloids on the periodic table. metalloids can also be called semimetals. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. They are brittle, poor conductors, and form amphoteric oxides. learn how to classify elements into three groups based on their properties and. Metalloids Are In What Group.
From studybeglerbegs.z4.web.core.windows.net
Where Are The Metalloids Periodic Table Metalloids Are In What Group Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. learn about the six metalloids in the periodic table: Learn about the location, list, and characteristics of metalloids on the periodic table. metalloids are elements that have properties intermediate between metals and nonmetals. Metals are shiny, conduct heat and electricity, and. learn. Metalloids Are In What Group.
From exoxachrg.blob.core.windows.net
Steel Is Element Compound Or Mixture at Corinne Rosales blog Metalloids Are In What Group learn about the six metalloids in the periodic table: the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Learn about the location, list, and characteristics of metalloids on the periodic table. Metals are shiny, conduct heat and electricity, and. Find out how they form covalent crystals, exhibit intermediate electronegativity,. Metalloids Are In What Group.
From sciencetrends.com
4 Properties Of Metalloids Science Trends Metalloids Are In What Group Boron, silicon, germanium, arsenic, antimony, and tellurium. metalloids can also be called semimetals. learn about the six metalloids in the periodic table: Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. Learn about the location, list, and characteristics of metalloids on the periodic table. Metals are shiny, conduct heat and. Metalloids Are In What Group.
From www.xometry.com
7 Elements of Metalloids Differences and Uses Xometry Metalloids Are In What Group They are brittle, poor conductors, and form amphoteric oxides. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Learn about the location, list, and characteristics of metalloids on the periodic table. Boron, silicon, germanium, arsenic, antimony, and tellurium. learn about the six metalloids in the periodic table: Metals are. Metalloids Are In What Group.
From edutechspot.com
Metalloids are located where on the periodic table? Here >>> Metalloids Are In What Group metalloids are elements that have properties intermediate between metals and nonmetals. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Metals are shiny, conduct heat and electricity, and. Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. learn about the seven metalloid. Metalloids Are In What Group.
From pediabay.com
Periodic Table Metals, Nonmetals & Metalloids Pediabay Metalloids Are In What Group Learn about the location, list, and characteristics of metalloids on the periodic table. They are brittle, poor conductors, and form amphoteric oxides. learn how to classify elements into three groups based on their properties and reactions. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Find out how they. Metalloids Are In What Group.
From scienceinfo.com
Metalloids Definition, Properties, Uses, and Applications Metalloids Are In What Group Metals are shiny, conduct heat and electricity, and. learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. the term is normally applied to a group of between six and nine elements (boron, silicon, germanium, arsenic,. Boron, silicon, germanium, arsenic, antimony, and tellurium. metalloids are elements that have properties intermediate between. Metalloids Are In What Group.
From brokeasshome.com
Metalloids Located On The Periodic Table Metalloids Are In What Group learn about the seven metalloid elements and their properties, uses, and locations on the periodic table. Metals are shiny, conduct heat and electricity, and. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. learn how to classify elements into three groups based on their properties and reactions. They are brittle,. Metalloids Are In What Group.
From www.haikudeck.com
Metalloids by Megan Maul Metalloids Are In What Group Metalloids are elements with intermediate characteristics of metals and nonmetals, such as semiconductivity and amphoterism. metalloids are elements that have properties intermediate between metals and nonmetals. Learn about the location, list, and characteristics of metalloids on the periodic table. Find out how they form covalent crystals, exhibit intermediate electronegativity, and have various oxidation states and applications. metalloids can. Metalloids Are In What Group.