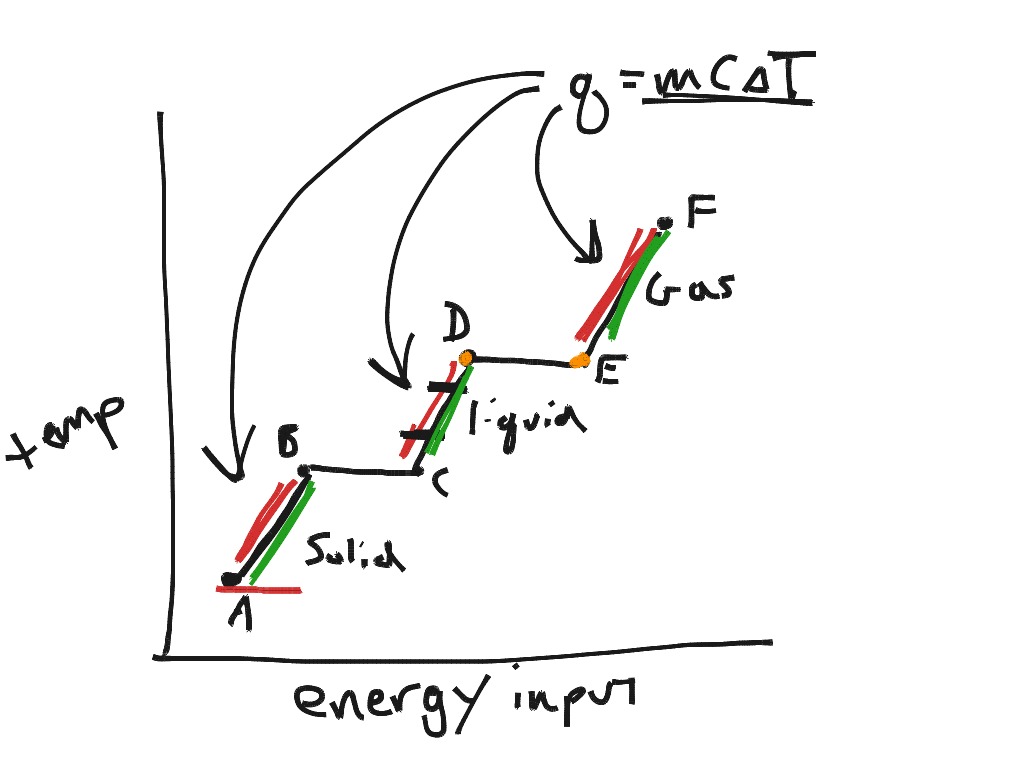
What Does C Stand For In Q=Mcat . C — specific heat capacity; The formula q = mct is used to calculate heat energy. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. M — mass of the substance; the equation $q = mc \delta t$ is appropriate for calorimetry at a constant volume. calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. There's 3 components to this question: q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. A very simple way to do this is. $q$ here refers to the net heat. Heat energy = mass × specific heat × change in. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mc∆t where:
from www.showme.com
q = mc∆t where: There's 3 components to this question: The formula q = mct is used to calculate heat energy. Heat energy = mass × specific heat × change in. M — mass of the substance; q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. the equation $q = mc \delta t$ is appropriate for calorimetry at a constant volume. calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction.
Understanding q=mcat Science, Chemistry ShowMe
What Does C Stand For In Q=Mcat calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. The formula q = mct is used to calculate heat energy. q = mc∆t where: q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. M — mass of the substance; A very simple way to do this is. C — specific heat capacity; Heat energy = mass × specific heat × change in. the equation $q = mc \delta t$ is appropriate for calorimetry at a constant volume. $q$ here refers to the net heat. calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. There's 3 components to this question:
From www.youtube.com
"Q=mcat" YouTube What Does C Stand For In Q=Mcat There's 3 components to this question: q = mc∆t where: The formula q = mct is used to calculate heat energy. calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c =. What Does C Stand For In Q=Mcat.
From fabalabse.com
What does C stand stand for? Leia aqui What does the C in Cstand What Does C Stand For In Q=Mcat q = mc∆t where: calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. The formula q = mct is used to calculate heat energy. q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in. What Does C Stand For In Q=Mcat.
From studylibrarygodward.z13.web.core.windows.net
How To Calculate Specific Heat Formula What Does C Stand For In Q=Mcat There's 3 components to this question: q = mc∆t where: q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. $q$ here refers to the net heat. Apply q reaction = (m) (c) (∆t), where delta t is an absolute. What Does C Stand For In Q=Mcat.
From fabalabse.com
What does C stand stand for? Leia aqui What does the C in Cstand What Does C Stand For In Q=Mcat A very simple way to do this is. q = mc∆t where: The formula q = mct is used to calculate heat energy. calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. $q$ here refers to the net heat. Apply q reaction = (m) (c) (∆t), where delta t. What Does C Stand For In Q=Mcat.
From mathuplift.com
What does c mean in math? What Does C Stand For In Q=Mcat q = mc∆t where: calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. C — specific heat capacity; Apply q. What Does C Stand For In Q=Mcat.
From www.youtube.com
2.0 Level q=mc∆T Practice YouTube What Does C Stand For In Q=Mcat Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. There's 3 components to this question: A very. What Does C Stand For In Q=Mcat.
From f0rmule.blogspot.com
Formule Q = M.c.delta T Formule What Does C Stand For In Q=Mcat $q$ here refers to the net heat. q = mc∆t where: There's 3 components to this question: q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. Apply q reaction = (m) (c) (∆t), where delta t is an absolute. What Does C Stand For In Q=Mcat.
From www.youtube.com
11.2 q=mCT YouTube What Does C Stand For In Q=Mcat $q$ here refers to the net heat. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. the equation $q = mc \delta t$ is appropriate for calorimetry at a constant volume. M — mass of the substance; q. What Does C Stand For In Q=Mcat.
From www.youtube.com
HTPIB14B Specific Heat and Q = mcT YouTube What Does C Stand For In Q=Mcat The formula q = mct is used to calculate heat energy. the equation $q = mc \delta t$ is appropriate for calorimetry at a constant volume. q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. There's 3 components to. What Does C Stand For In Q=Mcat.
From www.chegg.com
Solved Q mcAT Equation 2 1. Show that the units of specific What Does C Stand For In Q=Mcat The formula q = mct is used to calculate heat energy. $q$ here refers to the net heat. M — mass of the substance; Heat energy = mass × specific heat × change in. q = mc∆t where: calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. A very. What Does C Stand For In Q=Mcat.
From www.youtube.com
Specific Heat Capacity q = mcT Everything you need to know! Chemistry What Does C Stand For In Q=Mcat q = mc∆t where: $q$ here refers to the net heat. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in. What Does C Stand For In Q=Mcat.
From mcathub.com
The things you should know to get a good MCAT score What Does C Stand For In Q=Mcat $q$ here refers to the net heat. calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. the equation $q =. What Does C Stand For In Q=Mcat.
From fabalabse.com
What does the C stand for in credit? Leia aqui What does C mean in What Does C Stand For In Q=Mcat q = mc∆t where: Heat energy = mass × specific heat × change in. the equation $q = mc \delta t$ is appropriate for calorimetry at a constant volume. A very simple way to do this is. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially,. What Does C Stand For In Q=Mcat.
From exoiovlis.blob.core.windows.net
What Does C Stand For In A Car at Bruce Jagger blog What Does C Stand For In Q=Mcat q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. M —. What Does C Stand For In Q=Mcat.
From pametno21.blogspot.com
Q=Mcat Formula Chemistry pametno What Does C Stand For In Q=Mcat M — mass of the substance; A very simple way to do this is. C — specific heat capacity; Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical. What Does C Stand For In Q=Mcat.
From fabalabse.com
What does C stand stand for? Leia aqui What does the C in Cstand What Does C Stand For In Q=Mcat There's 3 components to this question: The formula q = mct is used to calculate heat energy. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. $q$ here refers to the net heat. Heat energy = mass × specific heat. What Does C Stand For In Q=Mcat.
From dxoasnubk.blob.core.windows.net
What Does Q T Stand For at Margie Fuller blog What Does C Stand For In Q=Mcat A very simple way to do this is. The formula q = mct is used to calculate heat energy. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. M — mass of the substance; There's 3 components to this question: $q$ here refers to the net. What Does C Stand For In Q=Mcat.
From magoosh.com
What is a Good MCAT Score? Magoosh MCAT Blog What Does C Stand For In Q=Mcat calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. A very simple way to do this is. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mc∆t q = heat energy (joules, j). What Does C Stand For In Q=Mcat.
From www.kaptest.com
What’s Tested on the MCAT (2024)? Kaplan Test Prep What Does C Stand For In Q=Mcat Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mc∆t where: The formula q = mct is used to calculate heat energy. Heat energy = mass × specific heat × change in. M — mass of the substance; calorimetry (q = mc. What Does C Stand For In Q=Mcat.
From www.showme.com
Solving q=mcat Science, Chemistry ShowMe What Does C Stand For In Q=Mcat q = mc∆t where: $q$ here refers to the net heat. There's 3 components to this question: q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. C — specific heat capacity; calorimetry (q = mc t) calorimetry is. What Does C Stand For In Q=Mcat.
From www.youtube.com
Q = mcΔT and Specific Heat IB Physics YouTube What Does C Stand For In Q=Mcat q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. Heat energy = mass × specific heat × change in. A very simple way to do this is. The formula q = mct is used to calculate heat energy. q. What Does C Stand For In Q=Mcat.
From fabalabse.com
What does C stand stand for? Leia aqui What does the C in Cstand What Does C Stand For In Q=Mcat q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. q = mc∆t where: $q$ here refers to the net heat. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always. What Does C Stand For In Q=Mcat.
From www.osmosis.org
Preparing for the MCAT Video, Anatomy & Definition Osmosis What Does C Stand For In Q=Mcat M — mass of the substance; calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. There's 3 components to this question:. What Does C Stand For In Q=Mcat.
From didyouknowfacts.com
What Does the "Q" in "QTip" Stand for? What Does C Stand For In Q=Mcat M — mass of the substance; There's 3 components to this question: q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. q = mc∆t where: C — specific heat capacity; Heat energy = mass × specific heat × change. What Does C Stand For In Q=Mcat.
From www.koodooslearning.com
Preparing for the MCAT? 8 Free Resources to Get You Started — Koodoos What Does C Stand For In Q=Mcat q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. There's 3 components to this question: $q$ here refers to the net heat. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is. What Does C Stand For In Q=Mcat.
From www.zdnet.com
What is the MCAT? What Does C Stand For In Q=Mcat There's 3 components to this question: C — specific heat capacity; q = mc∆t where: A very simple way to do this is. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mcδt where q is the thermal energy in j, c. What Does C Stand For In Q=Mcat.
From www.inspiraadvantage.com
What Is On The MCAT? A TestTakers Guide What Does C Stand For In Q=Mcat A very simple way to do this is. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the.. What Does C Stand For In Q=Mcat.
From www.showme.com
Understanding q=mcat Science, Chemistry ShowMe What Does C Stand For In Q=Mcat Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. $q$ here refers to the net heat. There's 3 components to this question: q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured. What Does C Stand For In Q=Mcat.
From www.wiringwork.com
what is q in q=mcat Wiring Work What Does C Stand For In Q=Mcat M — mass of the substance; the equation $q = mc \delta t$ is appropriate for calorimetry at a constant volume. calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always. What Does C Stand For In Q=Mcat.
From www.youtube.com
Calculating Heat Using Q=mcAT YouTube What Does C Stand For In Q=Mcat q = mc∆t where: q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. M — mass of the substance; A very simple way to do this is. the equation $q = mc \delta t$ is appropriate for calorimetry. What Does C Stand For In Q=Mcat.
From www.youtube.com
Using the formula q=mcΔT (Three examples) YouTube What Does C Stand For In Q=Mcat M — mass of the substance; the equation $q = mc \delta t$ is appropriate for calorimetry at a constant volume. $q$ here refers to the net heat. There's 3 components to this question: q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in. What Does C Stand For In Q=Mcat.
From exovcrumo.blob.core.windows.net
What Is The Formula For Calculating Distance In Physics at Genevieve What Does C Stand For In Q=Mcat q = mcδt where q is the thermal energy in j, c is the heat capacity, and δt is the temperature change measured in k, and m is. M — mass of the substance; The formula q = mct is used to calculate heat energy. $q$ here refers to the net heat. calorimetry (q = mc t) calorimetry. What Does C Stand For In Q=Mcat.
From www.mometrix.com
MCAT Test Prep (2024) MCAT Exam Review What Does C Stand For In Q=Mcat M — mass of the substance; There's 3 components to this question: $q$ here refers to the net heat. Apply q reaction = (m) (c) (∆t), where delta t is an absolute value so q reaction is always positive initially, then 2. A very simple way to do this is. C — specific heat capacity; the equation $q =. What Does C Stand For In Q=Mcat.
From quizlet.com
q=mCAT Diagram Quizlet What Does C Stand For In Q=Mcat C — specific heat capacity; q = mc∆t where: q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. Heat energy = mass × specific heat × change in. A very simple way to do this is. There's 3 components. What Does C Stand For In Q=Mcat.
From www.youtube.com
MCAT Question of the Day Specific Heat Calculations YouTube What Does C Stand For In Q=Mcat calorimetry (q = mc t) calorimetry is a way to measure the heat change of a chemical reaction. q = mc∆t where: There's 3 components to this question: q = mc∆t q = heat energy (joules, j) m = mass of a substance (kg) c = specific heat (units j/kg∙k) ∆ is a symbol meaning the. The. What Does C Stand For In Q=Mcat.