Will Nitrogen Oxide React With Water . although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. Hno 2 forms when n 2 o 3 reacts with water. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. But n 2 o and no are neutral gases. nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. It is more soluble in. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid;
from slidetodoc.com
nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. Hno 2 forms when n 2 o 3 reacts with water. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. It is more soluble in. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. But n 2 o and no are neutral gases. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react.
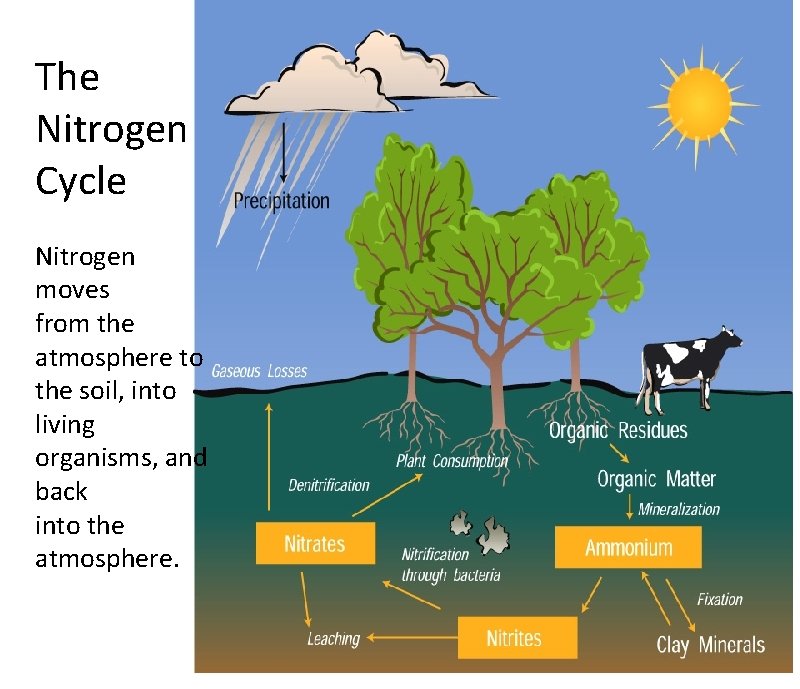
The Nitrogen Cycle Nitrogen like water is recycled
Will Nitrogen Oxide React With Water revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. But n 2 o and no are neutral gases. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. It is more soluble in. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. Hno 2 forms when n 2 o 3 reacts with water. nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and.
From www.slideserve.com
PPT NITROGENOXIDES PowerPoint Presentation, free download ID4735299 Will Nitrogen Oxide React With Water nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. compounds of. Will Nitrogen Oxide React With Water.
From www.pinterest.com
Chemistry Pals Laboratory Preparation of Nitrogen(I) Oxide Chemistry Will Nitrogen Oxide React With Water nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; Hno 2 forms when n 2 o 3 reacts with water. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. compounds of nitrogen (nitrates and nitrites) as well as nitrogen. Will Nitrogen Oxide React With Water.
From scienceinspiration.blogspot.com
Science Inspiration Acid Rain Causes Will Nitrogen Oxide React With Water nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. although. Will Nitrogen Oxide React With Water.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas And Oxygen Gas Reaction Will Nitrogen Oxide React With Water although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; nitrogen dioxide gas reacts with water. Will Nitrogen Oxide React With Water.
From fphoto.photoshelter.com
science chemical oxidation reaction nitrogen oxides Fundamental Will Nitrogen Oxide React With Water the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. But n 2 o and no are neutral gases. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. compounds of nitrogen (nitrates and nitrites). Will Nitrogen Oxide React With Water.
From kunduz.com
[ANSWERED] 1 Nitrogen dioxide and water react to produce nitric acid Will Nitrogen Oxide React With Water Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. It is more soluble in. Hno 2 forms when n 2 o 3 reacts with water. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; although nitric oxide is thermodynamically unstable, it is kinetically stable. Will Nitrogen Oxide React With Water.
From stock.adobe.com
Elements and Compounds are compared in the molecular structure. Oxygen Will Nitrogen Oxide React With Water compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. It is more soluble in. nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. But n 2 o and no are neutral gases. the aqueous solution solubility of no is 1.9 mm atm. Will Nitrogen Oxide React With Water.
From courses.lumenlearning.com
Classifying Chemical Reactions Chemistry Will Nitrogen Oxide React With Water But n 2 o and no are neutral gases. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. nitrogen dioxide gas reacts with water to produce nitric. Will Nitrogen Oxide React With Water.
From www.studyxapp.com
nitrogen monoxide and hydrogen react to form nitrogen and water like Will Nitrogen Oxide React With Water It is more soluble in. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. Hno 2 forms when n 2 o 3 reacts with water. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c. Will Nitrogen Oxide React With Water.
From www.chegg.com
Solved Ammonia, NH3, may react with oxygen to form nitrogen Will Nitrogen Oxide React With Water Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. But n 2 o and no are neutral gases. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very. Will Nitrogen Oxide React With Water.
From www.shutterstock.com
Acid Rain Is Caused By Emissions Of Sulfur Dioxide And Nitrogen Oxide Will Nitrogen Oxide React With Water the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. But n 2 o and no are neutral gases. Nitrogen dioxide(no 2) dissolves in water and. Will Nitrogen Oxide React With Water.
From www.chegg.com
Solved Nitrogen monoxide and oxygen react to form nitrogen Will Nitrogen Oxide React With Water compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide.. Will Nitrogen Oxide React With Water.
From www.algaebarn.com
Understanding the Nitrogen Cycle Beginners Education AlgaeBarn Will Nitrogen Oxide React With Water nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; Hno 2 forms when n 2 o 3 reacts with water. But n 2 o and no are neutral gases. although nitric oxide is thermodynamically unstable, it is kinetically stable as its. Will Nitrogen Oxide React With Water.
From www.bartleby.com
Answered Nitrogen monoxide and water react to… bartleby Will Nitrogen Oxide React With Water Hno 2 forms when n 2 o 3 reacts with water. It is more soluble in. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19].. Will Nitrogen Oxide React With Water.
From www.doubtnut.com
Nitric oxide reacts with hydrogen to give nitrogen and water 2NO +2H2 Will Nitrogen Oxide React With Water revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. Hno 2 forms when n 2 o 3 reacts with water. But n 2. Will Nitrogen Oxide React With Water.
From www.onlinebiologynotes.com
Nitrogen cycle Steps of Nitrogen cycle Online Biology Notes Will Nitrogen Oxide React With Water But n 2 o and no are neutral gases. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. nitrogen(iii) oxide, n 2 o 3, is the anhydride of. Will Nitrogen Oxide React With Water.
From www.science-sparks.com
What is the Nitrogen Cycle? Science for Kids Will Nitrogen Oxide React With Water Hno 2 forms when n 2 o 3 reacts with water. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and. Will Nitrogen Oxide React With Water.
From www.coursehero.com
[Solved] Nitrogen monoxide and water react to form ammonia and oxygen Will Nitrogen Oxide React With Water revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. But n 2 o and no are neutral gases. Nitrogen dioxide(no 2) dissolves in water. Will Nitrogen Oxide React With Water.
From water.unl.edu
Nitrogen Dynamics UNL Water Will Nitrogen Oxide React With Water the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. nitrogen(iii) oxide, n 2 o 3, is the anhydride of. Will Nitrogen Oxide React With Water.
From www.slideserve.com
PPT Types of Reactions and Combination Reactions PowerPoint Will Nitrogen Oxide React With Water Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref]. Will Nitrogen Oxide React With Water.
From socratic.org
How many grams of nitrogen dioxide must react with water to produce 5. Will Nitrogen Oxide React With Water Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. It is more soluble in. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. Hno 2 forms when n 2 o 3. Will Nitrogen Oxide React With Water.
From www.alamy.com
Acid rain is caused by emissions of sulfur dioxide and nitrogen oxide Will Nitrogen Oxide React With Water although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. It is more soluble in. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. But n 2 o and no are neutral gases. nitrogen. Will Nitrogen Oxide React With Water.
From www.chegg.com
Solved Nitric acid and nitrogen monoxide react to form Will Nitrogen Oxide React With Water nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. . Will Nitrogen Oxide React With Water.
From www.chegg.com
Solved Write the balanced chemical equation for the Will Nitrogen Oxide React With Water nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. Hno. Will Nitrogen Oxide React With Water.
From www.numerade.com
SOLVEDNitrogen dioxide and water react to produce nitric acid, HNO3 Will Nitrogen Oxide React With Water compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. But n 2 o and no are neutral gases. Hno 2 forms when n 2 o 3 reacts with water. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong. Will Nitrogen Oxide React With Water.
From www.chegg.com
Solved Nitrogen dioxide and water react to form nitric acid Will Nitrogen Oxide React With Water the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Nitrogen dioxide(no 2) dissolves in water and react with water to. Will Nitrogen Oxide React With Water.
From www.slideshare.net
Oxide of nitrogen Will Nitrogen Oxide React With Water revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written by the chemistry experts at save my exams. Hno 2 forms when n 2 o 3 reacts with water. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; But n 2 o and no are neutral gases. It is. Will Nitrogen Oxide React With Water.
From www.youtube.com
Chemical Reaction of Water with Nitrous Oxide, Chemistry Lecture Will Nitrogen Oxide React With Water Hno 2 forms when n 2 o 3 reacts with water. nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37. Will Nitrogen Oxide React With Water.
From slidetodoc.com
The Nitrogen Cycle Nitrogen like water is recycled Will Nitrogen Oxide React With Water although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; nitrogen dioxide gas reacts with. Will Nitrogen Oxide React With Water.
From www.numerade.com
SOLVED Nitrogen dioxide (NO2) gas and liquid water (H2O) react to form Will Nitrogen Oxide React With Water Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. It is more soluble in. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. although nitric oxide is thermodynamically unstable, it is kinetically stable. Will Nitrogen Oxide React With Water.
From www.numerade.com
SOLVED Nitrogen dioxide gas can react with water to form nitric acid Will Nitrogen Oxide React With Water compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not react. It is more soluble in. nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; But n 2 o and no are neutral gases. revision notes on 6.1.4 oxides reacting with water for the aqa. Will Nitrogen Oxide React With Water.
From www.chemistrystudent.com
Alkanes (ALevel) ChemistryStudent Will Nitrogen Oxide React With Water the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. It is more soluble in. revision notes on 6.1.4 oxides reacting with water for the aqa a level chemistry syllabus, written. Will Nitrogen Oxide React With Water.
From www.linstitute.net
CIE A Level Chemistry复习笔记2.1.3 Period 3 Oxides翰林国际教育 Will Nitrogen Oxide React With Water Hno 2 forms when n 2 o 3 reacts with water. But n 2 o and no are neutral gases. although nitric oxide is thermodynamically unstable, it is kinetically stable as its decomposition rate is very slow [ref] and. compounds of nitrogen (nitrates and nitrites) as well as nitrogen gas (n 2) dissolve in water but do not. Will Nitrogen Oxide React With Water.
From www.numerade.com
SOLVEDNitrogen dioxide and water react to produce nitric acid, HNO3 Will Nitrogen Oxide React With Water It is more soluble in. But n 2 o and no are neutral gases. Nitrogen dioxide(no 2) dissolves in water and react with water to give nitric acid(hno 3) which is a strong acid. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. compounds. Will Nitrogen Oxide React With Water.
From www.youtube.com
How to Balance NO2 + H2O = HNO2 + HNO3 (Nitrogen dioxide + Water) YouTube Will Nitrogen Oxide React With Water nitrogen(iii) oxide, n 2 o 3, is the anhydride of nitrous acid; It is more soluble in. the aqueous solution solubility of no is 1.9 mm atm −1 at 25 °c and 1.4 mm atm −1 at 37 °c [19]. nitrogen dioxide gas reacts with water to produce nitric acid and nitrogen monoxide. Nitrogen dioxide(no 2) dissolves. Will Nitrogen Oxide React With Water.