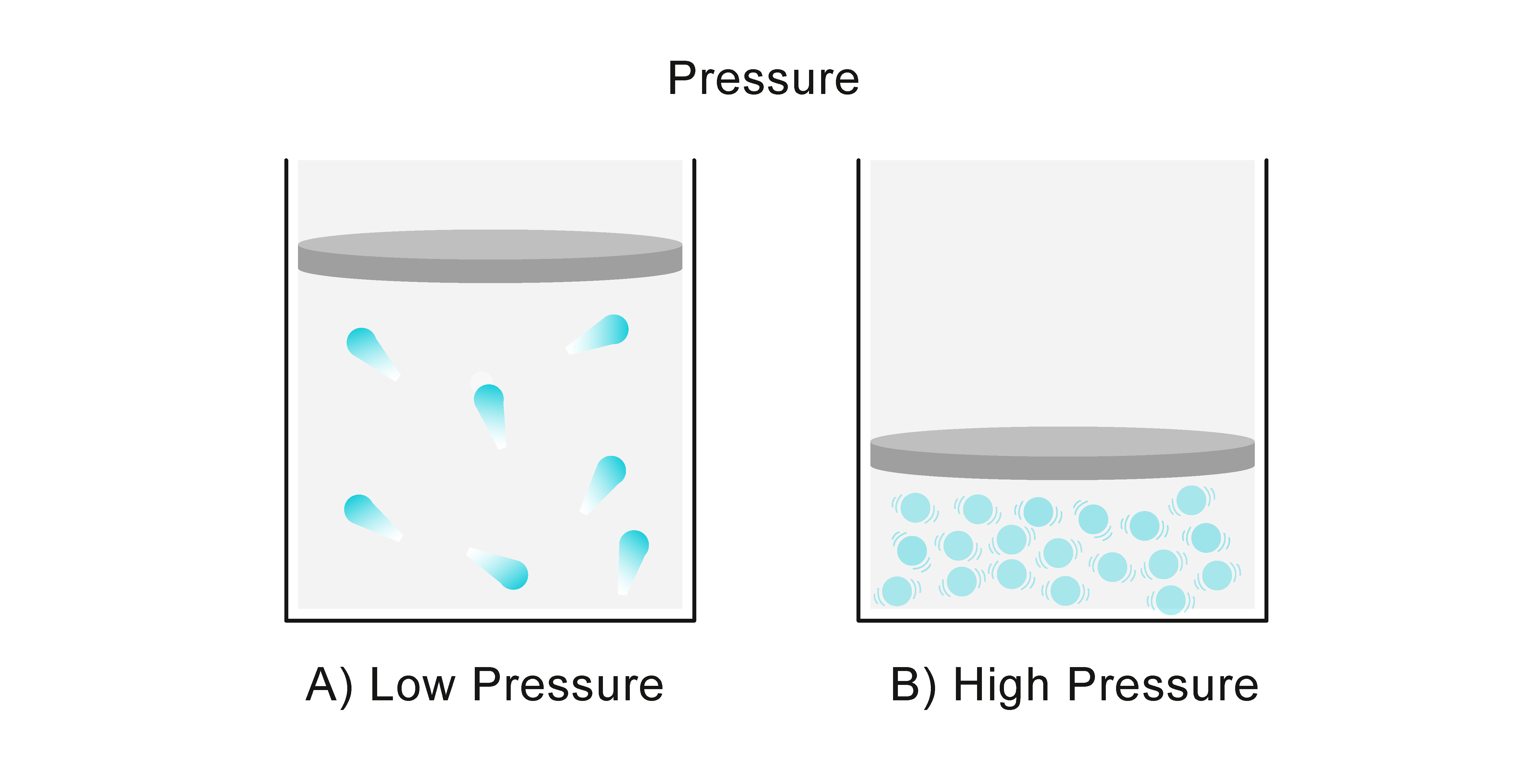
How Can Gas Pressure Be Increased . We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. How gas particles behave, and how that behaviour creates pressure ; They hit the walls of their container harder and more often,. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Although the force of each. Conversely, as the pressure on a gas decreases,. If a gas is heated, its particles move around more quickly.
from www.edplace.com
As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. How gas particles behave, and how that behaviour creates pressure ; Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Conversely, as the pressure on a gas decreases,. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Although the force of each. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. They hit the walls of their container harder and more often,. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). If a gas is heated, its particles move around more quickly.
Understand Gas Pressure Worksheet EdPlace
How Can Gas Pressure Be Increased As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Although the force of each. How gas particles behave, and how that behaviour creates pressure ; As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. If a gas is heated, its particles move around more quickly. They hit the walls of their container harder and more often,. Conversely, as the pressure on a gas decreases,. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)).
From www.tec-science.com
Specific heat capacity of gases (at constant volume or pressure) tec How Can Gas Pressure Be Increased We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. They hit the walls of their container harder and more often,. Although the force of each. Learn about. How Can Gas Pressure Be Increased.
From www.savemyexams.co.uk
Gas Law Relationships (1.2.5) IB DP Chemistry SL Revision Notes 2016 How Can Gas Pressure Be Increased Conversely, as the pressure on a gas decreases,. Although the force of each. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). As the pressure on a gas increases, the volume of. How Can Gas Pressure Be Increased.
From www.youtube.com
Effect of pressure Change of state from gas to liquid CBSE Class 9 How Can Gas Pressure Be Increased If a gas is heated, its particles move around more quickly. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. As the pressure on a gas increases, the volume. How Can Gas Pressure Be Increased.
From www.slideserve.com
PPT Gas Laws PowerPoint Presentation, free download ID818392 How Can Gas Pressure Be Increased As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. If a gas is heated, its particles move around more quickly. Although the force of each. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Conversely, as. How Can Gas Pressure Be Increased.
From www.slideserve.com
PPT Chapter 11 Gases PowerPoint Presentation, free download ID64297 How Can Gas Pressure Be Increased How gas particles behave, and how that behaviour creates pressure ; Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). If a gas is heated, its particles move around more quickly. Although the force of each. We will examine separately how the volume, temperature, and amount of gas each affect the. How Can Gas Pressure Be Increased.
From www.askiitians.com
Gas Laws And Properties Of Gases Study Material for IIT JEE askIITians How Can Gas Pressure Be Increased We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. How gas particles behave, and how that behaviour creates pressure ; Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. As the pressure on a gas increases, the volume. How Can Gas Pressure Be Increased.
From www.youtube.com
Constant Pressure Heating of a gas YouTube How Can Gas Pressure Be Increased As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Conversely, as. How Can Gas Pressure Be Increased.
From courses.lumenlearning.com
8.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas How Can Gas Pressure Be Increased Conversely, as the pressure on a gas decreases,. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. If a gas is heated, its particles move around more quickly. They. How Can Gas Pressure Be Increased.
From www.teachoo.com
Changing Pressure to Change State of Matter Chemistry Teachoo How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). How gas. How Can Gas Pressure Be Increased.
From saylordotorg.github.io
The Behavior of Real Gases How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. They hit the walls of their container harder and more often,. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Although the force of each. Conversely, as the pressure. How Can Gas Pressure Be Increased.
From www.slideserve.com
PPT Chapter 5 Gases PowerPoint Presentation, free download ID6918783 How Can Gas Pressure Be Increased They hit the walls of their container harder and more often,. As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). How gas particles behave, and how that behaviour creates pressure. How Can Gas Pressure Be Increased.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket How Can Gas Pressure Be Increased If a gas is heated, its particles move around more quickly. Although the force of each. As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Conversely, as the pressure on a gas decreases,. How gas particles behave, and how that behaviour creates pressure ; They hit the walls. How Can Gas Pressure Be Increased.
From www.youtube.com
Gases Pressure. YouTube How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. They hit the walls of their container harder and more often,. Although the force of each. Conversely, as the pressure on a. How Can Gas Pressure Be Increased.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock How Can Gas Pressure Be Increased How gas particles behave, and how that behaviour creates pressure ; Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. If a gas is heated, its particles move around more quickly. Conversely, as the pressure on a gas decreases,. As the pressure on a gas increases, the volume of the. How Can Gas Pressure Be Increased.
From masterconceptsinchemistry.com
As pressure increase the volume of gas decrease How Can Gas Pressure Be Increased Conversely, as the pressure on a gas decreases,. If a gas is heated, its particles move around more quickly. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. How. How Can Gas Pressure Be Increased.
From saylordotorg.github.io
Gases and Pressure How Can Gas Pressure Be Increased If a gas is heated, its particles move around more quickly. As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Conversely, as the pressure on a gas decreases,. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas. How Can Gas Pressure Be Increased.
From www.goodscience.com.au
Factors that Affect Rate of Reaction Good Science How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. If a gas is heated, its particles move around more quickly. How gas particles behave, and how that behaviour creates pressure ;. How Can Gas Pressure Be Increased.
From www.youtube.com
Gas Pressure and Volume GCSE Physics Revision YouTube How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. They hit the walls of their container harder and more often,. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). As the pressure on a gas increases, the volume of the gas. How Can Gas Pressure Be Increased.
From www.visionlearning.com
Properties of Gases Chemistry Visionlearning How Can Gas Pressure Be Increased How gas particles behave, and how that behaviour creates pressure ; We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Although the force of each. Conversely, as the pressure on a gas decreases,. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume. How Can Gas Pressure Be Increased.
From byjus.com
if for gas [ideal] if the pressure is increased by 10 How Can Gas Pressure Be Increased Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). If a gas is heated, its particles move around more quickly. They hit the walls of their container harder and more often,. Conversely, as the pressure on a gas decreases,. Although the force of each. Learn about and revise particle motion, gas. How Can Gas Pressure Be Increased.
From youtube.com
Calculating Gas Pressure Pressure in the Gas Laws YouTube How Can Gas Pressure Be Increased Although the force of each. Conversely, as the pressure on a gas decreases,. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. If a gas is heated, its particles move around more quickly. How gas particles behave, and how that behaviour creates pressure ; We will examine separately how the. How Can Gas Pressure Be Increased.
From www.tes.com
GCSEGas pressure and volume Teaching Resources How Can Gas Pressure Be Increased They hit the walls of their container harder and more often,. Although the force of each. How gas particles behave, and how that behaviour creates pressure ; Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). As the pressure on a gas increases, the volume of the gas decreases because the. How Can Gas Pressure Be Increased.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4144868 How Can Gas Pressure Be Increased As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. If a gas is heated, its particles move around more quickly. How gas particles behave, and how that behaviour creates pressure ; We will examine separately how the volume, temperature, and amount of gas each affect the pressure of. How Can Gas Pressure Be Increased.
From www.slideserve.com
PPT Compressibility PowerPoint Presentation, free download ID4200029 How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. How gas particles behave, and how that behaviour creates pressure ; They hit the walls of their container harder and. How Can Gas Pressure Be Increased.
From www.youtube.com
Ideal Gas Pressure and Volume Dependence on Temperature YouTube How Can Gas Pressure Be Increased How gas particles behave, and how that behaviour creates pressure ; Conversely, as the pressure on a gas decreases,. As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Although the force of each. If a gas is heated, its particles move around more quickly. They hit the walls. How Can Gas Pressure Be Increased.
From www.slideserve.com
PPT Chapter 14 Properties of Gases PowerPoint Presentation, free How Can Gas Pressure Be Increased Although the force of each. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. If a gas is heated, its particles move around more quickly. They hit the walls of their container harder and more often,. Conversely, as the pressure on a gas decreases,. Learn about and revise particle motion,. How Can Gas Pressure Be Increased.
From www.slideserve.com
PPT Chapter 17 Reaction PowerPoint Presentation, free How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Conversely,. How Can Gas Pressure Be Increased.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica How Can Gas Pressure Be Increased Although the force of each. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. They hit the walls of their container harder and more often,. Conversely, as the pressure on a gas decreases,. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects. How Can Gas Pressure Be Increased.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. If a gas is heated, its particles move around more quickly. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. They hit the walls of their container harder and. How Can Gas Pressure Be Increased.
From www.doubtnut.com
The pressure of a gas is increased 2 times. What should be the change How Can Gas Pressure Be Increased If a gas is heated, its particles move around more quickly. How gas particles behave, and how that behaviour creates pressure ; We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Conversely, as the pressure on a gas decreases,. Learn about and revise particle motion, gas pressure and. How Can Gas Pressure Be Increased.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources How Can Gas Pressure Be Increased Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Although the force of each. If a gas is heated, its particles move around more quickly. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Conversely, as the pressure. How Can Gas Pressure Be Increased.
From www.edplace.com
Understand Gas Pressure Worksheet EdPlace How Can Gas Pressure Be Increased Conversely, as the pressure on a gas decreases,. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. They hit the walls of their container harder and more often,. Gas pressure is caused by the force exerted by gas molecules colliding with the surfaces of objects ((figure)). How gas particles behave,. How Can Gas Pressure Be Increased.
From owlcation.com
The Theories and Behavior of Gas Owlcation How Can Gas Pressure Be Increased We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. How gas particles behave, and how that behaviour creates pressure ; Although the force of each. They hit the walls. How Can Gas Pressure Be Increased.
From gcsephysicsninja.com
11. Heating gas at a constant pressure How Can Gas Pressure Be Increased As the pressure on a gas increases, the volume of the gas decreases because the gas particles are forced closer together. Conversely, as the pressure on a gas decreases,. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Learn about and revise particle motion, gas pressure and the relationship between. How Can Gas Pressure Be Increased.
From saylordotorg.github.io
Gases How Can Gas Pressure Be Increased If a gas is heated, its particles move around more quickly. We will examine separately how the volume, temperature, and amount of gas each affect the pressure of an enclosed gas sample. Learn about and revise particle motion, gas pressure and the relationship between pressure and volume with gcse bitesize physics. Although the force of each. Gas pressure is caused. How Can Gas Pressure Be Increased.