Benefits Of Buffer . A solution whose ph is not altered to any great extent by the addition of small quantities of. a buffer is an aqueous solution that has a highly stable ph. Controlling selectivity on zwitterionic hilic. calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffers are characterized by the ph range over which they. for further information in this area, an article highlighting the importance of buffers is: Then, a second buffer (ph=4.0) is used to make fine. what is buffer in chemistry? a first buffer (commonly, ph=7.0) is used to make major adjustments; A buffering agent is a weak acid or weak base that helps maintain the ph of an. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.
from www.slideserve.com
A buffering agent is a weak acid or weak base that helps maintain the ph of an. a first buffer (commonly, ph=7.0) is used to make major adjustments; for further information in this area, an article highlighting the importance of buffers is: Then, a second buffer (ph=4.0) is used to make fine. calculate the amounts of acid and base needed to prepare a buffer of a given ph. a buffer is an aqueous solution that has a highly stable ph. Buffers are characterized by the ph range over which they. A solution whose ph is not altered to any great extent by the addition of small quantities of. what is buffer in chemistry? Controlling selectivity on zwitterionic hilic.
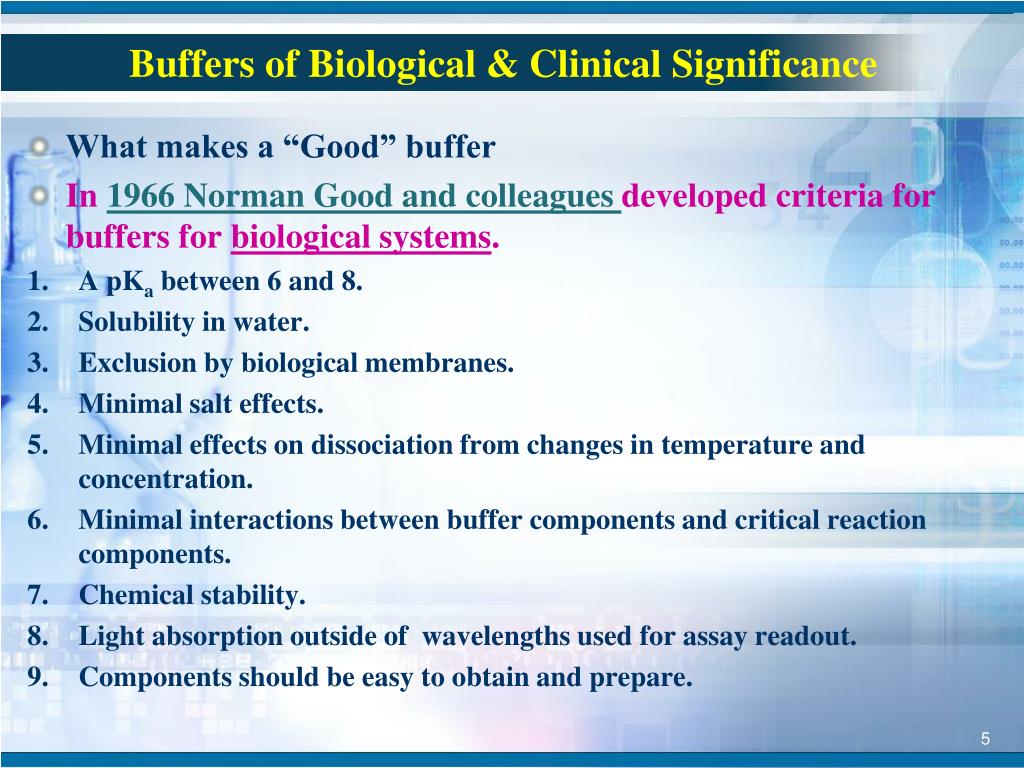
PPT Buffers of Biological & Clinical Significance PowerPoint
Benefits Of Buffer a first buffer (commonly, ph=7.0) is used to make major adjustments; what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is an aqueous solution that has a highly stable ph. calculate the amounts of acid and base needed to prepare a buffer of a given ph. Then, a second buffer (ph=4.0) is used to make fine. Controlling selectivity on zwitterionic hilic. Buffers are characterized by the ph range over which they. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist. for further information in this area, an article highlighting the importance of buffers is: A buffering agent is a weak acid or weak base that helps maintain the ph of an. a first buffer (commonly, ph=7.0) is used to make major adjustments;
From www.prinsco.com
When planning your buffer strips, consider the benefits of saturated Benefits Of Buffer a first buffer (commonly, ph=7.0) is used to make major adjustments; calculate the amounts of acid and base needed to prepare a buffer of a given ph. Controlling selectivity on zwitterionic hilic. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist. for further information in this area,. Benefits Of Buffer.
From www.see-the-opportunity.com
The Benefits Of A Floor Buffer SeeTheOpportunity Benefits Of Buffer Buffers are characterized by the ph range over which they. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution whose ph is not altered to any great extent by the addition of small quantities of. A buffering agent is a weak acid or weak. Benefits Of Buffer.
From www.slideserve.com
PPT Buffers of Biological & Clinical Significance PowerPoint Benefits Of Buffer A buffering agent is a weak acid or weak base that helps maintain the ph of an. a buffer is an aqueous solution that has a highly stable ph. calculate the amounts of acid and base needed to prepare a buffer of a given ph. Buffers are characterized by the ph range over which they. for further. Benefits Of Buffer.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG Benefits Of Buffer Controlling selectivity on zwitterionic hilic. A buffering agent is a weak acid or weak base that helps maintain the ph of an. a buffer is an aqueous solution that has a highly stable ph. for further information in this area, an article highlighting the importance of buffers is: a first buffer (commonly, ph=7.0) is used to make. Benefits Of Buffer.
From www.pinterest.com
What are the Buffers and its Importance? This article explains the Benefits Of Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. Then, a second buffer (ph=4.0) is used to make fine. for further information in this area, an article highlighting the importance of buffers is: Controlling selectivity on zwitterionic hilic. a buffer is an aqueous solution. Benefits Of Buffer.
From www.jamesriverbuffers.org
Why buffers matter the James River Buffer Program JAMES RIVER Benefits Of Buffer Buffers are characterized by the ph range over which they. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. for further information in this area, an article highlighting the importance of buffers is: buffer solutions are incredibly useful as they have the ability to. Benefits Of Buffer.
From buffer.com
Buffer State Of Remote Work 2023 Benefits Of Buffer what is buffer in chemistry? Controlling selectivity on zwitterionic hilic. a first buffer (commonly, ph=7.0) is used to make major adjustments; Then, a second buffer (ph=4.0) is used to make fine. A solution whose ph is not altered to any great extent by the addition of small quantities of. buffer solutions are incredibly useful as they have. Benefits Of Buffer.
From news.aroutfitting.com
Quick Tip Benefits of an A5 Buffer in Your AR15 ARO News Benefits Of Buffer a first buffer (commonly, ph=7.0) is used to make major adjustments; for further information in this area, an article highlighting the importance of buffers is: A buffering agent is a weak acid or weak base that helps maintain the ph of an. a buffer is an aqueous solution that has a highly stable ph. Controlling selectivity on. Benefits Of Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Benefits Of Buffer Then, a second buffer (ph=4.0) is used to make fine. a buffer is an aqueous solution that has a highly stable ph. Buffers are characterized by the ph range over which they. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist. A buffering agent is a weak acid or. Benefits Of Buffer.
From learn.apolloheatpumps.com
Supercharge Your Heating System Exploring the Benefits of Buffer Tanks Benefits Of Buffer calculate the amounts of acid and base needed to prepare a buffer of a given ph. Controlling selectivity on zwitterionic hilic. what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. a first buffer (commonly, ph=7.0) is used to make major adjustments; a. Benefits Of Buffer.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Benefits Of Buffer for further information in this area, an article highlighting the importance of buffers is: Controlling selectivity on zwitterionic hilic. what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. a first buffer (commonly, ph=7.0) is used to make major adjustments; buffer solutions are. Benefits Of Buffer.
From www.slideserve.com
PPT Chem. Concepts Buffers PowerPoint Presentation, free download Benefits Of Buffer A buffering agent is a weak acid or weak base that helps maintain the ph of an. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. what is buffer in chemistry? Then, a second buffer (ph=4.0) is used to make fine. for further information. Benefits Of Buffer.
From vetmarketingpro.com
5 Benefits of Using Buffer Benefits Of Buffer A solution whose ph is not altered to any great extent by the addition of small quantities of. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases.. Benefits Of Buffer.
From www.slideserve.com
PPT Trees for the Bay PowerPoint Presentation, free download ID2466898 Benefits Of Buffer Then, a second buffer (ph=4.0) is used to make fine. for further information in this area, an article highlighting the importance of buffers is: a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is an aqueous solution that has a highly stable. Benefits Of Buffer.
From sciencing.com
Characteristics of Good Buffers Sciencing Benefits Of Buffer calculate the amounts of acid and base needed to prepare a buffer of a given ph. a buffer is an aqueous solution that has a highly stable ph. Controlling selectivity on zwitterionic hilic. what is buffer in chemistry? for further information in this area, an article highlighting the importance of buffers is: A solution whose ph. Benefits Of Buffer.
From prismpub.com
What are riparian buffer strips? PRISM Benefits Of Buffer a buffer is an aqueous solution that has a highly stable ph. for further information in this area, an article highlighting the importance of buffers is: Then, a second buffer (ph=4.0) is used to make fine. a first buffer (commonly, ph=7.0) is used to make major adjustments; a buffer is a solution that maintains the stability. Benefits Of Buffer.
From keystogetwealth.com
A Comprehensive Guide to Buffer Social Media Management Tool Benefits Of Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. calculate the amounts of acid and base needed to prepare a buffer of a given ph. A solution whose ph is not altered to any great extent by the addition of small quantities of. Buffers are. Benefits Of Buffer.
From www.ethika.co.in
What is Corporate Buffer in Group Health Insurance? Benefits Of Buffer a buffer is an aqueous solution that has a highly stable ph. for further information in this area, an article highlighting the importance of buffers is: what is buffer in chemistry? Then, a second buffer (ph=4.0) is used to make fine. Buffers are characterized by the ph range over which they. A solution whose ph is not. Benefits Of Buffer.
From www.musimmas.com
How Do Riparian Buffers Protect the Environment? Musim Mas Benefits Of Buffer a first buffer (commonly, ph=7.0) is used to make major adjustments; what is buffer in chemistry? A solution whose ph is not altered to any great extent by the addition of small quantities of. A buffering agent is a weak acid or weak base that helps maintain the ph of an. a buffer is an aqueous solution. Benefits Of Buffer.
From www.slideserve.com
PPT Agroforestry II Riparian Buffers PowerPoint Presentation, free Benefits Of Buffer a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a buffer is an aqueous solution that has a highly stable ph. Then, a second buffer (ph=4.0) is used to make fine. for further information in this area, an article highlighting the importance of buffers. Benefits Of Buffer.
From livingonthebank.com
Riparian Buffer Living on the Bank Benefits Of Buffer Then, a second buffer (ph=4.0) is used to make fine. calculate the amounts of acid and base needed to prepare a buffer of a given ph. A buffering agent is a weak acid or weak base that helps maintain the ph of an. a buffer is an aqueous solution that has a highly stable ph. what is. Benefits Of Buffer.
From beautymedicaldevices.com
What is Miracle Body Buffer and the Benefits of this Ultimate Body Tool? Benefits Of Buffer calculate the amounts of acid and base needed to prepare a buffer of a given ph. A buffering agent is a weak acid or weak base that helps maintain the ph of an. for further information in this area, an article highlighting the importance of buffers is: Buffers are characterized by the ph range over which they. Then,. Benefits Of Buffer.
From bwsr.state.mn.us
Buffer and Alternative Practices Benefits MN Board of Water, Soil Benefits Of Buffer A solution whose ph is not altered to any great extent by the addition of small quantities of. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist.. Benefits Of Buffer.
From www.slideshare.net
Buffers in chemical analysis, types of buffers Benefits Of Buffer for further information in this area, an article highlighting the importance of buffers is: calculate the amounts of acid and base needed to prepare a buffer of a given ph. Controlling selectivity on zwitterionic hilic. what is buffer in chemistry? buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance. Benefits Of Buffer.
From es.slideshare.net
Uses of buffers Benefits Of Buffer a first buffer (commonly, ph=7.0) is used to make major adjustments; Controlling selectivity on zwitterionic hilic. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. A solution whose ph is not altered to any great extent by the addition of small quantities of. Then, a. Benefits Of Buffer.
From www.youtube.com
how buffer solution acts / importance of buffer /why we need buffer Benefits Of Buffer Controlling selectivity on zwitterionic hilic. a buffer is an aqueous solution that has a highly stable ph. what is buffer in chemistry? a first buffer (commonly, ph=7.0) is used to make major adjustments; calculate the amounts of acid and base needed to prepare a buffer of a given ph. a buffer is a solution that. Benefits Of Buffer.
From bufferoptionsnh.org
Buffer Basics Buffer Options for the Bay Benefits Of Buffer a first buffer (commonly, ph=7.0) is used to make major adjustments; a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist. A solution whose ph is not. Benefits Of Buffer.
From www.youtube.com
Why Everyone Should Have A Cash Buffer (Surprising Benefits of Cash Benefits Of Buffer Buffers are characterized by the ph range over which they. Controlling selectivity on zwitterionic hilic. A buffering agent is a weak acid or weak base that helps maintain the ph of an. for further information in this area, an article highlighting the importance of buffers is: buffer solutions are incredibly useful as they have the ability to maintain. Benefits Of Buffer.
From www.planetanalog.com
Understanding the benefits of precharge buffers in ADCs Analog Benefits Of Buffer calculate the amounts of acid and base needed to prepare a buffer of a given ph. Controlling selectivity on zwitterionic hilic. a buffer is an aqueous solution that has a highly stable ph. for further information in this area, an article highlighting the importance of buffers is: a first buffer (commonly, ph=7.0) is used to make. Benefits Of Buffer.
From www.slideserve.com
PPT Watershed Management Framework PowerPoint Presentation, free Benefits Of Buffer a first buffer (commonly, ph=7.0) is used to make major adjustments; A buffering agent is a weak acid or weak base that helps maintain the ph of an. Then, a second buffer (ph=4.0) is used to make fine. calculate the amounts of acid and base needed to prepare a buffer of a given ph. what is buffer. Benefits Of Buffer.
From www.slideserve.com
PPT Best Management Practices on the Golf Course PowerPoint Benefits Of Buffer for further information in this area, an article highlighting the importance of buffers is: A solution whose ph is not altered to any great extent by the addition of small quantities of. Controlling selectivity on zwitterionic hilic. a first buffer (commonly, ph=7.0) is used to make major adjustments; Then, a second buffer (ph=4.0) is used to make fine.. Benefits Of Buffer.
From www4.des.state.nh.us
The Importance of Riparian Buffers New Hampshire Local River Benefits Of Buffer a first buffer (commonly, ph=7.0) is used to make major adjustments; Controlling selectivity on zwitterionic hilic. Buffers are characterized by the ph range over which they. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist. A buffering agent is a weak acid or weak base that helps maintain the. Benefits Of Buffer.
From thechemistrynotes.com
Buffer Solution Types, Properties, and Uses Benefits Of Buffer A solution whose ph is not altered to any great extent by the addition of small quantities of. Controlling selectivity on zwitterionic hilic. a buffer is a solution that maintains the stability of a system’s ph level when adding small quantities of acids or bases. a first buffer (commonly, ph=7.0) is used to make major adjustments; what. Benefits Of Buffer.
From buffer.com
Key Insights from The 2023 State of Remote Work Benefits Of Buffer a buffer is an aqueous solution that has a highly stable ph. A buffering agent is a weak acid or weak base that helps maintain the ph of an. what is buffer in chemistry? Then, a second buffer (ph=4.0) is used to make fine. Controlling selectivity on zwitterionic hilic. Buffers are characterized by the ph range over which. Benefits Of Buffer.
From www.allianceforthebay.org
Strengthening and Building "Buffer" Partnerships Alliance for the Benefits Of Buffer calculate the amounts of acid and base needed to prepare a buffer of a given ph. buffer solutions are incredibly useful as they have the ability to maintain a stable ph balance and resist. Controlling selectivity on zwitterionic hilic. a first buffer (commonly, ph=7.0) is used to make major adjustments; what is buffer in chemistry? . Benefits Of Buffer.