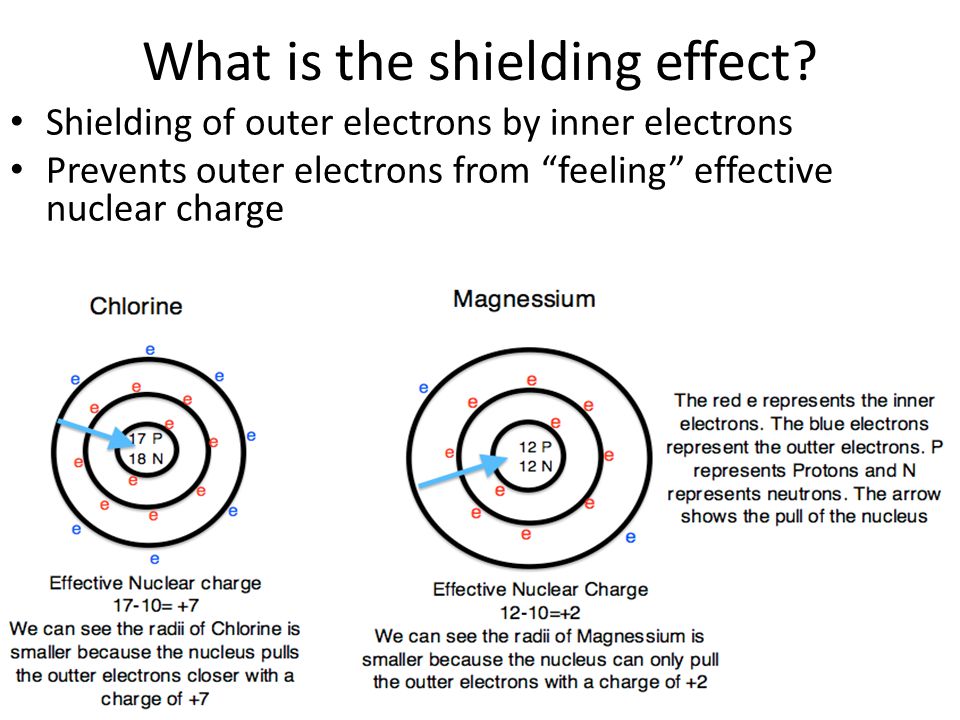
How Does Electron Shielding Effect Ionization Energy . going down a group, the ionisation energy decreases. Therefore, using coulomb’s law, one. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding and ionization energy. what influence does a larger shielding effect have on ionization energy? How do s orbit electrons affect the. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. A hydrogen atom has no shielding effect since it has only one electron.
from socratic.org
A hydrogen atom has no shielding effect since it has only one electron. How do s orbit electrons affect the. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. what influence does a larger shielding effect have on ionization energy? Therefore, using coulomb’s law, one. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. electron shielding and ionization energy. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. going down a group, the ionisation energy decreases.
How are shielding effect and atomic radius related? Socratic
How Does Electron Shielding Effect Ionization Energy How do s orbit electrons affect the. Therefore, using coulomb’s law, one. electron shielding and ionization energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. How do s orbit electrons affect the. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. what influence does a larger shielding effect have on ionization energy? A hydrogen atom has no shielding effect since it has only one electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. going down a group, the ionisation energy decreases.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free How Does Electron Shielding Effect Ionization Energy Ionization energy is the energy required to remove an electron from a neutral gaseous atom. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. A hydrogen atom has no shielding effect since it has only one electron. How do s orbit electrons affect the. This. How Does Electron Shielding Effect Ionization Energy.
From slideplayer.com
Periodic Trends Section ppt download How Does Electron Shielding Effect Ionization Energy electron shielding and ionization energy. How do s orbit electrons affect the. Therefore, using coulomb’s law, one. A hydrogen atom has no shielding effect since it has only one electron. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. what influence does a larger shielding effect. How Does Electron Shielding Effect Ionization Energy.
From surfguppy.com
Ionization energy trend Surfguppy Chemistry made easy for visual How Does Electron Shielding Effect Ionization Energy the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding and ionization energy. Therefore, using coulomb’s law, one. going down a group, the ionisation energy decreases. A hydrogen atom has no shielding effect since it has only one electron. the concept. How Does Electron Shielding Effect Ionization Energy.
From exozhcqdr.blob.core.windows.net
What Is Shielding Effect Simple Definition at Dawn Cook blog How Does Electron Shielding Effect Ionization Energy what influence does a larger shielding effect have on ionization energy? A hydrogen atom has no shielding effect since it has only one electron. electron shielding and ionization energy. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. This is due to the shielding or screen effect of the outer electrons from. How Does Electron Shielding Effect Ionization Energy.
From educationfruits.z21.web.core.windows.net
What Is Shielding Effect In Chemistry How Does Electron Shielding Effect Ionization Energy going down a group, the ionisation energy decreases. electron shielding and ionization energy. How do s orbit electrons affect the. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. what influence does a larger shielding effect have on ionization energy? Ionization energy is the energy. How Does Electron Shielding Effect Ionization Energy.
From slideplayer.com
Periodic Trends, Cont. Shielding Ion Size Ionization Energy How Does Electron Shielding Effect Ionization Energy what influence does a larger shielding effect have on ionization energy? Therefore, using coulomb’s law, one. going down a group, the ionisation energy decreases. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. How Does Electron Shielding Effect Ionization Energy.
From www.slideserve.com
PPT Drill 6 11/11 & 11/12/13 PowerPoint Presentation, free download How Does Electron Shielding Effect Ionization Energy A hydrogen atom has no shielding effect since it has only one electron. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Therefore, using. How Does Electron Shielding Effect Ionization Energy.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive How Does Electron Shielding Effect Ionization Energy going down a group, the ionisation energy decreases. what influence does a larger shielding effect have on ionization energy? This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. A hydrogen atom has no shielding effect since it has only one electron. the concept of electron. How Does Electron Shielding Effect Ionization Energy.
From www.slideserve.com
PPT IONISATION ENERGY PowerPoint Presentation, free download ID3500637 How Does Electron Shielding Effect Ionization Energy Ionization energy is the energy required to remove an electron from a neutral gaseous atom. A hydrogen atom has no shielding effect since it has only one electron. going down a group, the ionisation energy decreases. How do s orbit electrons affect the. Therefore, using coulomb’s law, one. the concept of electron shielding, in which intervening electrons act. How Does Electron Shielding Effect Ionization Energy.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity How Does Electron Shielding Effect Ionization Energy A hydrogen atom has no shielding effect since it has only one electron. going down a group, the ionisation energy decreases. Therefore, using coulomb’s law, one. what influence does a larger shielding effect have on ionization energy? the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. How Does Electron Shielding Effect Ionization Energy.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e How Does Electron Shielding Effect Ionization Energy electron shielding and ionization energy. what influence does a larger shielding effect have on ionization energy? Therefore, using coulomb’s law, one. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. This is due to the shielding or screen effect of the outer electrons. How Does Electron Shielding Effect Ionization Energy.
From slideplayer.com
Periodic Trends, Cont. Shielding Ion Size Ionization Energy How Does Electron Shielding Effect Ionization Energy Therefore, using coulomb’s law, one. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear. How Does Electron Shielding Effect Ionization Energy.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog How Does Electron Shielding Effect Ionization Energy going down a group, the ionisation energy decreases. Therefore, using coulomb’s law, one. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. A hydrogen atom has no shielding effect since it has only one electron. This is due to the shielding or screen effect. How Does Electron Shielding Effect Ionization Energy.
From www.youtube.com
Atomic radius, size, Ionization Energy, Electron Affinity How Does Electron Shielding Effect Ionization Energy Ionization energy is the energy required to remove an electron from a neutral gaseous atom. Therefore, using coulomb’s law, one. A hydrogen atom has no shielding effect since it has only one electron. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. what influence does a larger. How Does Electron Shielding Effect Ionization Energy.
From slideplayer.com
Atomic Radii Ionic Radii Ionization Energies Electronegativity ppt How Does Electron Shielding Effect Ionization Energy going down a group, the ionisation energy decreases. How do s orbit electrons affect the. A hydrogen atom has no shielding effect since it has only one electron. electron shielding and ionization energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. This. How Does Electron Shielding Effect Ionization Energy.
From slideplayer.com
Periodic Trends Chemistry. ppt download How Does Electron Shielding Effect Ionization Energy Ionization energy is the energy required to remove an electron from a neutral gaseous atom. Therefore, using coulomb’s law, one. what influence does a larger shielding effect have on ionization energy? This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. the concept of electron shielding, in. How Does Electron Shielding Effect Ionization Energy.
From www.slideserve.com
PPT Unit 2 20142015 PowerPoint Presentation, free download ID How Does Electron Shielding Effect Ionization Energy How do s orbit electrons affect the. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. A hydrogen atom has no shielding effect since it has only one electron. what influence does a larger shielding effect have on ionization energy? going down a. How Does Electron Shielding Effect Ionization Energy.
From socratic.org
How are shielding effect and atomic radius related? Socratic How Does Electron Shielding Effect Ionization Energy This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. electron shielding and ionization energy. going down a group, the ionisation energy decreases. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.. How Does Electron Shielding Effect Ionization Energy.
From www.studypool.com
SOLUTION Properties of periodicity atomic size and radius shielding How Does Electron Shielding Effect Ionization Energy electron shielding and ionization energy. going down a group, the ionisation energy decreases. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. the concept of electron shielding,. How Does Electron Shielding Effect Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps How Does Electron Shielding Effect Ionization Energy going down a group, the ionisation energy decreases. Therefore, using coulomb’s law, one. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. what influence does a larger shielding effect have on ionization energy? A hydrogen atom has no shielding effect since it has. How Does Electron Shielding Effect Ionization Energy.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube How Does Electron Shielding Effect Ionization Energy Therefore, using coulomb’s law, one. electron shielding and ionization energy. A hydrogen atom has no shielding effect since it has only one electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. what influence does a larger shielding effect have on ionization energy?. How Does Electron Shielding Effect Ionization Energy.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download How Does Electron Shielding Effect Ionization Energy the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. electron shielding and ionization energy. going down a group, the ionisation energy decreases. How do s orbit electrons affect the. Therefore, using coulomb’s law, one. Ionization energy is the energy required to remove an. How Does Electron Shielding Effect Ionization Energy.
From www.studypool.com
SOLUTION Properties of periodicity atomic size and radius shielding How Does Electron Shielding Effect Ionization Energy the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. This is due to the shielding or screen effect of the outer. How Does Electron Shielding Effect Ionization Energy.
From ar.inspiredpencil.com
Shielding Effect Electrons How Does Electron Shielding Effect Ionization Energy A hydrogen atom has no shielding effect since it has only one electron. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. going down a group, the ionisation energy decreases. what. How Does Electron Shielding Effect Ionization Energy.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples How Does Electron Shielding Effect Ionization Energy going down a group, the ionisation energy decreases. electron shielding and ionization energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. This is due to the shielding. How Does Electron Shielding Effect Ionization Energy.
From www.youtube.com
Atomic Radius Ionization Energy Electron AffinityElectronegativity How Does Electron Shielding Effect Ionization Energy what influence does a larger shielding effect have on ionization energy? This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. electron shielding and ionization energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron,. How Does Electron Shielding Effect Ionization Energy.
From educationfruits.z21.web.core.windows.net
What Is Shielding Effect In Chemistry How Does Electron Shielding Effect Ionization Energy what influence does a larger shielding effect have on ionization energy? This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Ionization energy is. How Does Electron Shielding Effect Ionization Energy.
From www.youtube.com
what is ionization energy?Shielding effect Trend in periodic table How Does Electron Shielding Effect Ionization Energy A hydrogen atom has no shielding effect since it has only one electron. Therefore, using coulomb’s law, one. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. what influence. How Does Electron Shielding Effect Ionization Energy.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint How Does Electron Shielding Effect Ionization Energy A hydrogen atom has no shielding effect since it has only one electron. Therefore, using coulomb’s law, one. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. going down a group, the ionisation energy decreases. what influence does a larger shielding effect have on ionization energy?. How Does Electron Shielding Effect Ionization Energy.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The How Does Electron Shielding Effect Ionization Energy A hydrogen atom has no shielding effect since it has only one electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. How do s orbit electrons affect the. Therefore, using coulomb’s law, one. Ionization energy is the energy required to remove an electron from. How Does Electron Shielding Effect Ionization Energy.
From www.slideserve.com
PPT Chapter 6 Ionic Bonds and Some Main Group Chemistry PowerPoint How Does Electron Shielding Effect Ionization Energy going down a group, the ionisation energy decreases. electron shielding and ionization energy. How do s orbit electrons affect the. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. This is due to the shielding or screen effect of the outer electrons from the nucleus and so the attraction is. what. How Does Electron Shielding Effect Ionization Energy.
From general.chemistrysteps.com
Ionization energy Chemistry Steps How Does Electron Shielding Effect Ionization Energy A hydrogen atom has no shielding effect since it has only one electron. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the. How Does Electron Shielding Effect Ionization Energy.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e How Does Electron Shielding Effect Ionization Energy Ionization energy is the energy required to remove an electron from a neutral gaseous atom. How do s orbit electrons affect the. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. what influence does a larger shielding effect have on ionization energy? going. How Does Electron Shielding Effect Ionization Energy.
From www.studypool.com
SOLUTION Properties of periodicity atomic size and radius shielding How Does Electron Shielding Effect Ionization Energy electron shielding and ionization energy. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. A hydrogen atom has no shielding effect since it has only one electron. Ionization energy is the energy required to remove an electron from a neutral gaseous atom. How do. How Does Electron Shielding Effect Ionization Energy.
From slideplayer.com
Periodic Trends, Cont. Shielding Ion Size Ionization Energy How Does Electron Shielding Effect Ionization Energy Ionization energy is the energy required to remove an electron from a neutral gaseous atom. what influence does a larger shielding effect have on ionization energy? the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. going down a group, the ionisation energy decreases.. How Does Electron Shielding Effect Ionization Energy.