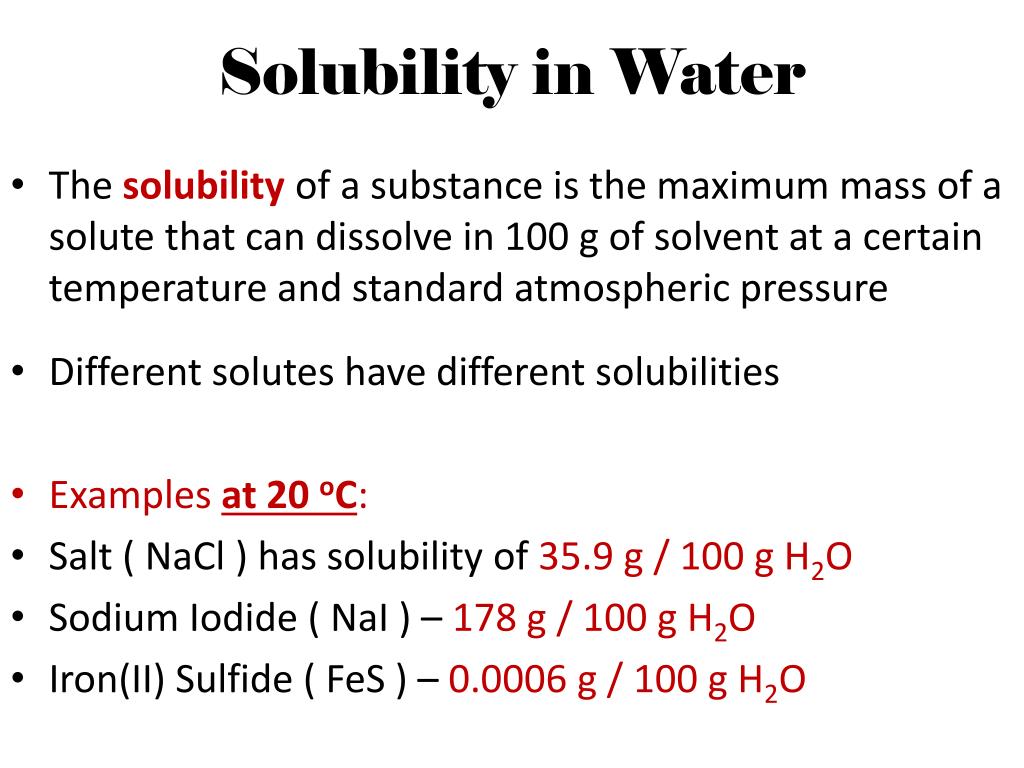
Solid In Liquid Solubility . In the first, the pure solid is melted at. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. To understand this, the dissolution of a solid can be visualized as occurring in two steps: Solutions of solids in liquids. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in.
from www.slideserve.com
Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. In the first, the pure solid is melted at. The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. To understand this, the dissolution of a solid can be visualized as occurring in two steps: Solutions of solids in liquids. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in.
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID
Solid In Liquid Solubility Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. In the first, the pure solid is melted at. To understand this, the dissolution of a solid can be visualized as occurring in two steps: Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. Solutions of solids in liquids. The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions.
From www.slideserve.com
PPT Solubility PowerPoint Presentation ID5581895 Solid In Liquid Solubility Solutions of solids in liquids. In the first, the pure solid is melted at. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions.. Solid In Liquid Solubility.
From www.youtube.com
Solubility of solids in liquids YouTube Solid In Liquid Solubility Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. To understand this, the dissolution of a solid can be visualized as occurring in two steps: The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Learn how temperature,. Solid In Liquid Solubility.
From www.youtube.com
Properties of Solids, Liquids and Gases YouTube Solid In Liquid Solubility To understand this, the dissolution of a solid can be visualized as occurring in two steps: In the first, the pure solid is melted at. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids,. Solid In Liquid Solubility.
From slidetodoc.com
Solubility equilibrium Solubility Property of a solid liquid Solid In Liquid Solubility In the first, the pure solid is melted at. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. To understand this, the dissolution of a solid can be visualized as occurring. Solid In Liquid Solubility.
From www.slideserve.com
PPT Chapter 1213 Mixtures and Aqueous Solutions PowerPoint Solid In Liquid Solubility Solutions of solids in liquids. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. To understand this, the dissolution of a solid can be visualized as occurring in two steps: Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's. Solid In Liquid Solubility.
From infogram.com
Solubility Infographic Infogram Solid In Liquid Solubility The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Solutions of solids in liquids. The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. In the first, the pure solid is melted at. Learn. Solid In Liquid Solubility.
From chem.libretexts.org
13.4 Pressure and Temperature Effects on Solubility Chemistry LibreTexts Solid In Liquid Solubility Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. In the first, the pure solid is melted at. Solutions of solids in liquids. To understand this, the dissolution of. Solid In Liquid Solubility.
From www.slideserve.com
PPT Substances, Mixtures, and Solubility PowerPoint Presentation Solid In Liquid Solubility Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Solutions of solids in liquids. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. To understand this, the dissolution of a solid can be visualized as occurring in. Solid In Liquid Solubility.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Solid In Liquid Solubility Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how. Solid In Liquid Solubility.
From www.slideserve.com
PPT Chapter Substances, Mixtures, and Solubility PowerPoint Solid In Liquid Solubility Solutions of solids in liquids. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. In the first, the pure solid is melted at. Learn. Solid In Liquid Solubility.
From www.slideserve.com
PPT Measuring solubility of solids PowerPoint Presentation, free Solid In Liquid Solubility Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Solutions of solids in liquids. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to. Solid In Liquid Solubility.
From www.youtube.com
Factors Affecting Solubility of Solid in Liquid Solution and Solid In Liquid Solubility To understand this, the dissolution of a solid can be visualized as occurring in two steps: Solutions of solids in liquids. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and. Solid In Liquid Solubility.
From animalia-life.club
Example Of Soluble Solid In Liquid Solubility To understand this, the dissolution of a solid can be visualized as occurring in two steps: In the first, the pure solid is melted at. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. The dependence of solubility on temperature for a number of solids in water is. Solid In Liquid Solubility.
From www.youtube.com
Factors affecting solubility of Solid in a Liquid Solution chapters 1 Solid In Liquid Solubility The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. To understand this, the dissolution of a solid can be visualized as occurring in two steps: Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Learn about the. Solid In Liquid Solubility.
From chem.libretexts.org
Solubility and Precipitation Chemistry LibreTexts Solid In Liquid Solubility The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Solutions of solids in liquids. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids,. Solid In Liquid Solubility.
From psiberg.com
Properties of Solid, Liquid, Gases A Comparison Solid In Liquid Solubility The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. To understand this, the dissolution of a solid can be visualized as occurring in. Solid In Liquid Solubility.
From www.slideserve.com
PPT Solubility Notes PowerPoint Presentation, free download ID5606676 Solid In Liquid Solubility Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Solutions of solids in liquids. To understand this, the dissolution of a solid can be visualized as occurring in two steps: The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Learn about. Solid In Liquid Solubility.
From www.docsity.com
Solubility General Chemistry Lecture Slides Docsity Solid In Liquid Solubility To understand this, the dissolution of a solid can be visualized as occurring in two steps: In the first, the pure solid is melted at. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and. Solid In Liquid Solubility.
From www.pinterest.com.au
Soluble Easy Science Easy science, Science student, Science rules Solid In Liquid Solubility In the first, the pure solid is melted at. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. The dependence of solubility on temperature for. Solid In Liquid Solubility.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science Solid In Liquid Solubility To understand this, the dissolution of a solid can be visualized as occurring in two steps: The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves. Solid In Liquid Solubility.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? Solid In Liquid Solubility Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. In the first, the pure solid is melted at. Solutions of solids in liquids. The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the. Solid In Liquid Solubility.
From www.askiitians.com
Classification of Solids Study Material for IIT JEE askIITians Solid In Liquid Solubility The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Solutions of. Solid In Liquid Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Solid In Liquid Solubility In the first, the pure solid is melted at. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. Learn about the different types of solutions, the effects of temperature. Solid In Liquid Solubility.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Solid In Liquid Solubility The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. In the first, the pure solid is melted at. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Learn about the factors affecting. Solid In Liquid Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID6260427 Solid In Liquid Solubility Solutions of solids in liquids. In the first, the pure solid is melted at. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. The solubility of a salt hydrate in water is. Solid In Liquid Solubility.
From www.youtube.com
Temperature and Solubility Solids and Gases YouTube Solid In Liquid Solubility The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. To understand this, the dissolution of a solid can be visualized as occurring in two steps: Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Learn about the. Solid In Liquid Solubility.
From www.youtube.com
solubility solutions class 12 chemistry subject notes lectures cbse Solid In Liquid Solubility The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. To understand this, the dissolution of a solid can be visualized as occurring in two. Solid In Liquid Solubility.
From fr.dreamstime.com
Solution Solide Dans Le Liquide Illustration de Vecteur Illustration Solid In Liquid Solubility Solutions of solids in liquids. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. The dependence of solubility on temperature for a number of solids in water. Solid In Liquid Solubility.
From www.slideserve.com
PPT Intermolecular Forces PowerPoint Presentation ID705859 Solid In Liquid Solubility To understand this, the dissolution of a solid can be visualized as occurring in two steps: In the first, the pure solid is melted at. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's law and raoult's law. The solubility of a salt hydrate in water is usually given as. Solid In Liquid Solubility.
From www.youtube.com
SOLUBILITY SOLUBILITY OF SOLID IN LIQUID SOLUTIONS PART 8 Solid In Liquid Solubility Solutions of solids in liquids. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. To understand this, the dissolution of a solid can be visualized as occurring in two steps: Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's. Solid In Liquid Solubility.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Solid In Liquid Solubility Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Solutions of solids in liquids. In the first, the pure solid is melted at. To understand this, the dissolution of a solid. Solid In Liquid Solubility.
From www.breakingatom.com
Solubility of Elements and Compounds Solid In Liquid Solubility Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. Solutions of solids in liquids. In the first, the pure solid is melted at. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Learn about the different types of solutions, the effects. Solid In Liquid Solubility.
From www.dreamstime.com
Dissolving Solids. Solubility Chemistry Stock Vector Illustration of Solid In Liquid Solubility Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. The dependence of solubility on temperature for a number of solids in water is shown by the solubility curves in. Learn about the factors affecting the solubility of gases, liquids, and solids in liquids, and how to apply henry's. Solid In Liquid Solubility.
From classnotes.org.in
Solubility of Gases and Solids in Liquids Chemistry, Class 12, Solutions Solid In Liquid Solubility The solubility of a salt hydrate in water is usually given as the relative proportion of anhydrous salt in solution, rather than the relative proportions. Solutions of solids in liquids. To understand this, the dissolution of a solid can be visualized as occurring in two steps: In the first, the pure solid is melted at. Learn about the factors affecting. Solid In Liquid Solubility.
From www.slideserve.com
PPT Solubility PowerPoint Presentation, free download ID37453 Solid In Liquid Solubility Learn about the different types of solutions, the effects of temperature and pressure on solubility, and how to use solubility curves. Learn how temperature, pressure, and common ions affect the solubility of solids, liquids, and gases in water. To understand this, the dissolution of a solid can be visualized as occurring in two steps: Solutions of solids in liquids. Learn. Solid In Liquid Solubility.