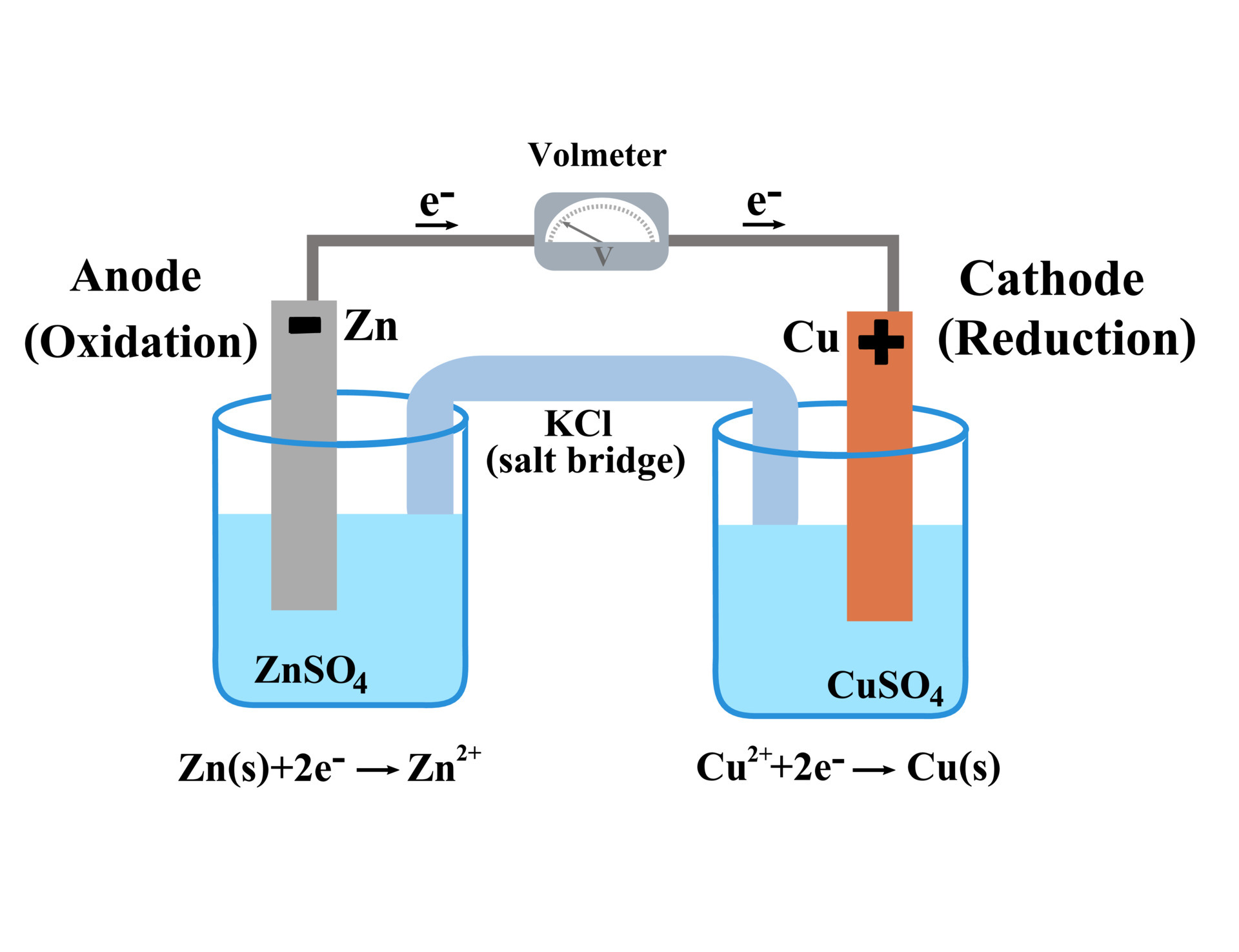
Where Does Oxidation Occur In The Electrochemical Cell . Reduction describes the gain of electrons by a molecule, atom, or ion. Nonspontaneous reactions occur in electrolytic cells. Spontaneous reactions occur in galvanic (voltaic) cells; Both types of cells contain electrodes where the oxidation. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The electrons always flow from the anode to the cathode. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Oxidation describes the loss of electrons by a molecule, atom, or ion.
from www.vecteezy.com
By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Spontaneous reactions occur in galvanic (voltaic) cells; Reduction describes the gain of electrons by a molecule, atom, or ion. Oxidation describes the loss of electrons by a molecule, atom, or ion. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. The electrons always flow from the anode to the cathode. Nonspontaneous reactions occur in electrolytic cells. Both types of cells contain electrodes where the oxidation.
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and
Where Does Oxidation Occur In The Electrochemical Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. Nonspontaneous reactions occur in electrolytic cells. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Spontaneous reactions occur in galvanic (voltaic) cells; Both types of cells contain electrodes where the oxidation. The electrons always flow from the anode to the cathode. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Reduction describes the gain of electrons by a molecule, atom, or ion. Oxidation describes the loss of electrons by a molecule, atom, or ion.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 Where Does Oxidation Occur In The Electrochemical Cell Reduction describes the gain of electrons by a molecule, atom, or ion. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The electrons always flow from the anode to the cathode. The two compartments of an electrochemical cell where the half reactions occur. Where Does Oxidation Occur In The Electrochemical Cell.
From www.slideserve.com
PPT Electrochemical Cells (voltaic cells) PowerPoint Presentation Where Does Oxidation Occur In The Electrochemical Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Oxidation describes the loss of electrons by a molecule, atom, or ion. Reduction describes the gain of electrons by a molecule, atom, or ion. Nonspontaneous reactions occur in electrolytic cells. The two compartments of. Where Does Oxidation Occur In The Electrochemical Cell.
From www.researchgate.net
The mechanism of electrochemical oxidation involved for the removal of Where Does Oxidation Occur In The Electrochemical Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Reduction describes the gain of electrons by a molecule, atom, or ion. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must. Where Does Oxidation Occur In The Electrochemical Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Where Does Oxidation Occur In The Electrochemical Cell The electrons always flow from the anode to the cathode. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Oxidation describes the loss of electrons by a molecule, atom, or ion. Reduction describes the gain of electrons by a molecule, atom, or ion. Nonspontaneous reactions. Where Does Oxidation Occur In The Electrochemical Cell.
From learningcampusstall.z21.web.core.windows.net
Electrochemical Cells A Level Chemistry Where Does Oxidation Occur In The Electrochemical Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode. Where Does Oxidation Occur In The Electrochemical Cell.
From merinyjoreenmatias.weebly.com
TASK 2 OXIDATION AND REDUCTION PRINCIPLES IN BIOCHEMISTRY Where Does Oxidation Occur In The Electrochemical Cell Spontaneous reactions occur in galvanic (voltaic) cells; The electrons always flow from the anode to the cathode. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Nonspontaneous reactions occur in electrolytic cells. Both types of cells contain electrodes where the oxidation. Oxidation describes the loss. Where Does Oxidation Occur In The Electrochemical Cell.
From taneshalg-images.blogspot.com
Oxidation Layers Of Learning Science Hands On Experiments Family Where Does Oxidation Occur In The Electrochemical Cell Reduction describes the gain of electrons by a molecule, atom, or ion. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The electrons always flow from the anode to the cathode. Both types of cells contain electrodes where the oxidation. Oxidation describes the. Where Does Oxidation Occur In The Electrochemical Cell.
From www.numerade.com
SOLVED Identify the location of oxidation in an electrochemical cell Where Does Oxidation Occur In The Electrochemical Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. Both types of cells contain electrodes where the oxidation. Spontaneous reactions occur in galvanic (voltaic) cells; Nonspontaneous reactions occur in electrolytic cells. The electrons always flow from the anode to the cathode. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in. Where Does Oxidation Occur In The Electrochemical Cell.
From edenmeowbeard.blogspot.com
Redox Reaction Meaning Where Does Oxidation Occur In The Electrochemical Cell The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. The electrons always flow from the anode to the cathode. Spontaneous reactions occur in galvanic (voltaic) cells; By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the. Where Does Oxidation Occur In The Electrochemical Cell.
From classfullbooklice.z13.web.core.windows.net
Working Of Electrochemical Cell Where Does Oxidation Occur In The Electrochemical Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Spontaneous reactions occur in galvanic (voltaic) cells; Reduction describes the gain of electrons by a molecule, atom, or ion. The two compartments of an electrochemical cell where the half reactions occur are called the. Where Does Oxidation Occur In The Electrochemical Cell.
From www.youtube.com
Half cell, oxidation half cell,reduction half cell(Electrochemistry Where Does Oxidation Occur In The Electrochemical Cell Reduction describes the gain of electrons by a molecule, atom, or ion. Oxidation describes the loss of electrons by a molecule, atom, or ion. Nonspontaneous reactions occur in electrolytic cells. The electrons always flow from the anode to the cathode. Spontaneous reactions occur in galvanic (voltaic) cells; The two compartments of an electrochemical cell where the half reactions occur are. Where Does Oxidation Occur In The Electrochemical Cell.
From chemwiki.ucdavis.edu
Voltaic Cells Chemwiki Where Does Oxidation Occur In The Electrochemical Cell Nonspontaneous reactions occur in electrolytic cells. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Spontaneous reactions occur in galvanic (voltaic) cells; Both types of cells contain electrodes where the oxidation. The electrons always flow from the anode to the cathode. Reduction describes. Where Does Oxidation Occur In The Electrochemical Cell.
From www.researchgate.net
Schematic diagram of the electrochemical cell for wastewater Where Does Oxidation Occur In The Electrochemical Cell Nonspontaneous reactions occur in electrolytic cells. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Reduction describes the gain of electrons by a molecule, atom, or ion. Oxidation describes the loss of electrons by a molecule, atom, or ion. Spontaneous reactions occur in. Where Does Oxidation Occur In The Electrochemical Cell.
From www.researchgate.net
The electrooxidation process involves electron (e⁻) transfer and in Where Does Oxidation Occur In The Electrochemical Cell Spontaneous reactions occur in galvanic (voltaic) cells; Nonspontaneous reactions occur in electrolytic cells. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The electrons always flow from the anode to the cathode. Both types of cells contain electrodes where the oxidation. Reduction describes. Where Does Oxidation Occur In The Electrochemical Cell.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Where Does Oxidation Occur In The Electrochemical Cell Spontaneous reactions occur in galvanic (voltaic) cells; Both types of cells contain electrodes where the oxidation. Reduction describes the gain of electrons by a molecule, atom, or ion. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. The electrons always flow from the anode to. Where Does Oxidation Occur In The Electrochemical Cell.
From www.chegg.com
Solved Where does oxidation occur in an electrochemical Where Does Oxidation Occur In The Electrochemical Cell Nonspontaneous reactions occur in electrolytic cells. Both types of cells contain electrodes where the oxidation. The electrons always flow from the anode to the cathode. Spontaneous reactions occur in galvanic (voltaic) cells; By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The two. Where Does Oxidation Occur In The Electrochemical Cell.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Where Does Oxidation Occur In The Electrochemical Cell Spontaneous reactions occur in galvanic (voltaic) cells; The electrons always flow from the anode to the cathode. Both types of cells contain electrodes where the oxidation. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Reduction describes the gain of electrons by a molecule, atom,. Where Does Oxidation Occur In The Electrochemical Cell.
From stock.adobe.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Where Does Oxidation Occur In The Electrochemical Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The electrons always flow from the anode to the cathode. Nonspontaneous reactions occur in electrolytic cells. Reduction describes the gain of electrons by a molecule, atom, or ion. Spontaneous reactions occur in galvanic (voltaic). Where Does Oxidation Occur In The Electrochemical Cell.
From www.chemistrystudent.com
Electrochemistry (ALevel) ChemistryStudent Where Does Oxidation Occur In The Electrochemical Cell Nonspontaneous reactions occur in electrolytic cells. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Reduction describes the gain of electrons by a molecule, atom, or ion. Spontaneous reactions occur in galvanic (voltaic) cells; Both types of cells contain electrodes where the oxidation. By definition,. Where Does Oxidation Occur In The Electrochemical Cell.
From pendulumedu.com
Oxidation and Reduction Redox Reactions, Definitions, Examples Where Does Oxidation Occur In The Electrochemical Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Spontaneous reactions occur in galvanic (voltaic) cells; Reduction describes the gain of electrons by a molecule, atom, or ion. Nonspontaneous reactions occur. Where Does Oxidation Occur In The Electrochemical Cell.
From theedge.com.hk
Chemistry How To Electrochemical Cell The Edge Where Does Oxidation Occur In The Electrochemical Cell Spontaneous reactions occur in galvanic (voltaic) cells; Reduction describes the gain of electrons by a molecule, atom, or ion. Oxidation describes the loss of electrons by a molecule, atom, or ion. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The two compartments. Where Does Oxidation Occur In The Electrochemical Cell.
From www.researchgate.net
Mechanism of electrochemical oxidation [115]. Download Scientific Diagram Where Does Oxidation Occur In The Electrochemical Cell Nonspontaneous reactions occur in electrolytic cells. Reduction describes the gain of electrons by a molecule, atom, or ion. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Oxidation describes the loss of electrons by a molecule, atom, or ion. Spontaneous reactions occur in. Where Does Oxidation Occur In The Electrochemical Cell.
From www.researchgate.net
A typical electrochemical CO2 reduction reaction cell. The oxidation Where Does Oxidation Occur In The Electrochemical Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Spontaneous reactions occur in galvanic (voltaic) cells; The electrons always flow from the anode to the cathode. By definition, the anode of an electrochemical. Where Does Oxidation Occur In The Electrochemical Cell.
From www.researchgate.net
Schematic diagram illustrating the electrochemical oxidation mechanism Where Does Oxidation Occur In The Electrochemical Cell The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Spontaneous reactions occur in galvanic (voltaic) cells; Both types of cells contain electrodes where the oxidation. Reduction describes the gain of electrons by a molecule, atom, or ion. By definition, the anode of an electrochemical cell. Where Does Oxidation Occur In The Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry OxidationReduction Reactions PowerPoint Where Does Oxidation Occur In The Electrochemical Cell Nonspontaneous reactions occur in electrolytic cells. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Spontaneous reactions occur in galvanic (voltaic) cells; By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the. Where Does Oxidation Occur In The Electrochemical Cell.
From slidetodoc.com
Chapter 14 Notes Fundamentals of Electrochemistry Redox Reactions Where Does Oxidation Occur In The Electrochemical Cell Both types of cells contain electrodes where the oxidation. Nonspontaneous reactions occur in electrolytic cells. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Spontaneous reactions occur in galvanic (voltaic) cells; Oxidation describes the loss of electrons by a molecule, atom, or ion. By definition,. Where Does Oxidation Occur In The Electrochemical Cell.
From courses.lumenlearning.com
Oxidative Phosphorylation OpenStax Biology 2e Where Does Oxidation Occur In The Electrochemical Cell Nonspontaneous reactions occur in electrolytic cells. Spontaneous reactions occur in galvanic (voltaic) cells; The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Oxidation describes the loss of electrons by a molecule, atom, or ion. The electrons always flow from the anode to the cathode. Reduction. Where Does Oxidation Occur In The Electrochemical Cell.
From www.thoughtco.com
How to Define Anode and Cathode Where Does Oxidation Occur In The Electrochemical Cell Oxidation describes the loss of electrons by a molecule, atom, or ion. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Nonspontaneous reactions occur in electrolytic cells. Spontaneous reactions occur in galvanic (voltaic) cells; Reduction describes the gain of electrons by a molecule, atom, or. Where Does Oxidation Occur In The Electrochemical Cell.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Where Does Oxidation Occur In The Electrochemical Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. The electrons always flow from the anode to the cathode.. Where Does Oxidation Occur In The Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID5744606 Where Does Oxidation Occur In The Electrochemical Cell By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Reduction describes the gain of electrons by a molecule, atom, or ion. Nonspontaneous reactions occur in electrolytic cells. Oxidation describes the loss of electrons by a molecule, atom, or ion. Both types of cells. Where Does Oxidation Occur In The Electrochemical Cell.
From ero-blog.com
At Which Electrode Does Oxidation Take Place Where Does Oxidation Occur In The Electrochemical Cell Spontaneous reactions occur in galvanic (voltaic) cells; By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. Oxidation describes the loss of electrons by a molecule, atom, or ion. The electrons always flow from the anode to the cathode. The two compartments of an. Where Does Oxidation Occur In The Electrochemical Cell.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Where Does Oxidation Occur In The Electrochemical Cell Nonspontaneous reactions occur in electrolytic cells. Oxidation describes the loss of electrons by a molecule, atom, or ion. The electrons always flow from the anode to the cathode. Spontaneous reactions occur in galvanic (voltaic) cells; The two compartments of an electrochemical cell where the half reactions occur are called the anode and the cathode, and they must have an. Reduction. Where Does Oxidation Occur In The Electrochemical Cell.
From slidetodoc.com
Aim How do electrochemical cells use redox reactions Where Does Oxidation Occur In The Electrochemical Cell The electrons always flow from the anode to the cathode. Nonspontaneous reactions occur in electrolytic cells. Reduction describes the gain of electrons by a molecule, atom, or ion. Oxidation describes the loss of electrons by a molecule, atom, or ion. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu. Where Does Oxidation Occur In The Electrochemical Cell.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID889382 Where Does Oxidation Occur In The Electrochemical Cell Spontaneous reactions occur in galvanic (voltaic) cells; Nonspontaneous reactions occur in electrolytic cells. Oxidation describes the loss of electrons by a molecule, atom, or ion. The electrons always flow from the anode to the cathode. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is. Where Does Oxidation Occur In The Electrochemical Cell.
From 2012books.lardbucket.org
Describing Electrochemical Cells Where Does Oxidation Occur In The Electrochemical Cell Reduction describes the gain of electrons by a molecule, atom, or ion. Both types of cells contain electrodes where the oxidation. By definition, the anode of an electrochemical cell is the electrode at which oxidation occurs (in this case, the cu foil) and the cathode is the. The electrons always flow from the anode to the cathode. Oxidation describes the. Where Does Oxidation Occur In The Electrochemical Cell.