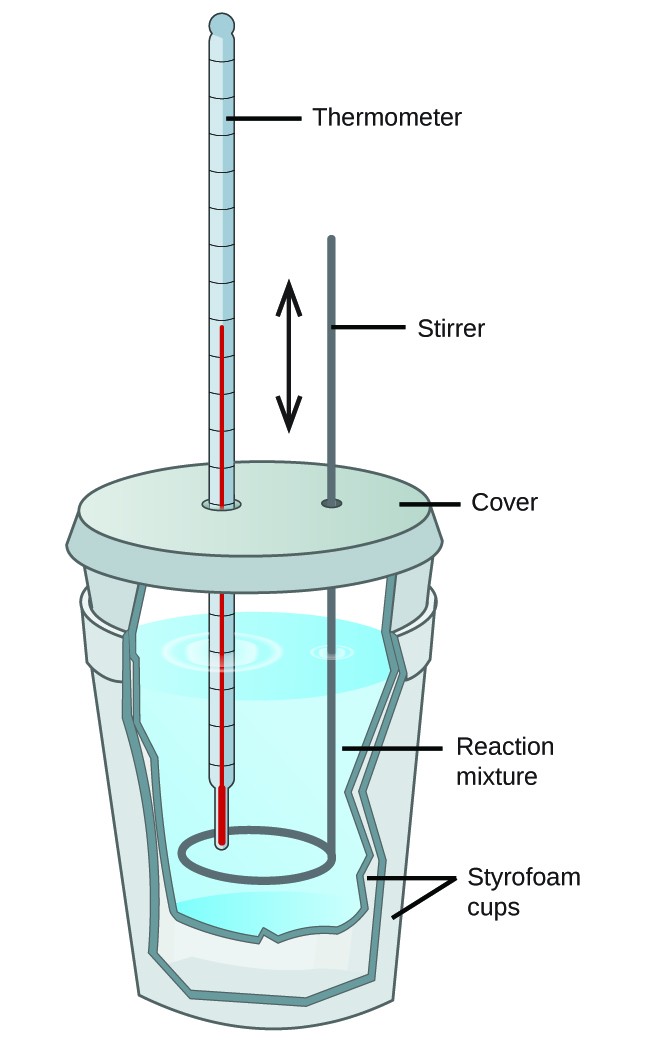
What Makes A Good Calorimeter . the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. Δt is the change in the temperature. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. C is the specific heat of the body. To do so, the heat is exchanged with a calibrated object. The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases in temperature. — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we have material of a uniform composition, we can relate the heat transferred from the hot object to the cold object by eq. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. Q is the measure of heat transfer. M is the mass of the body. — heat lost = heat gained. some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. calorimetry is used to measure amounts of heat transferred to or from a substance.
from courses.lumenlearning.com
a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. C is the specific heat of the body. — heat lost = heat gained. — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we have material of a uniform composition, we can relate the heat transferred from the hot object to the cold object by eq. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. Δt is the change in the temperature. calorimetry is used to measure amounts of heat transferred to or from a substance. Q is the measure of heat transfer. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials.
Calorimetry Chemistry for Majors
What Makes A Good Calorimeter Q is the measure of heat transfer. Δt is the change in the temperature. — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we have material of a uniform composition, we can relate the heat transferred from the hot object to the cold object by eq. C is the specific heat of the body. some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases in temperature. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. — heat lost = heat gained. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is used to measure amounts of heat transferred to or from a substance. Q is the measure of heat transfer. M is the mass of the body. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. To do so, the heat is exchanged with a calibrated object.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types What Makes A Good Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Δt is the. What Makes A Good Calorimeter.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Makes A Good Calorimeter Δt is the change in the temperature. C is the specific heat of the body. M is the mass of the body. calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object. Q is the measure of heat transfer. the most common types. What Makes A Good Calorimeter.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement What Makes A Good Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. some of the most common types include reaction calorimeters, scanning. What Makes A Good Calorimeter.
From pressbooks.calstate.edu
3.1 Calorimetry Nutrition and Physical Fitness What Makes A Good Calorimeter The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Q is the measure of heat transfer. the most common types of calorimeters are differential scanning calorimeters,. What Makes A Good Calorimeter.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas What Makes A Good Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Q is the measure of heat transfer. C is the specific heat of the body. — heat lost = heat gained. — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we. What Makes A Good Calorimeter.
From gamesmartz.com
Calorimeter Definition Easy to Understand What Makes A Good Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Δt is the change in the temperature. some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. calorimetry is used to measure amounts of heat transferred to or from a substance. . What Makes A Good Calorimeter.
From saylordotorg.github.io
Calorimetry What Makes A Good Calorimeter C is the specific heat of the body. — heat lost = heat gained. — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we have material of a uniform composition, we can relate the heat transferred from the hot object to the cold object by eq. calorimeter, device for measuring. What Makes A Good Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Makes A Good Calorimeter Q is the measure of heat transfer. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta. What Makes A Good Calorimeter.
From www.youtube.com
Principle of Calorimetry YouTube What Makes A Good Calorimeter — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we have material of a uniform composition, we can relate the heat transferred from the hot object to the cold object by eq. calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a. What Makes A Good Calorimeter.
From www.youtube.com
Thermochemistry ConstantVolume Calorimeter (Bomb Calorimeter). YouTube What Makes A Good Calorimeter — heat lost = heat gained. Δt is the change in the temperature. some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. C is the specific heat of the body. To do so, the heat is exchanged with a calibrated object. The heat transfer in a system is calculated using the formula, \ (\begin. What Makes A Good Calorimeter.
From www.animalia-life.club
Calorimeter Diagram What Makes A Good Calorimeter Q is the measure of heat transfer. calorimetry is used to measure amounts of heat transferred to or from a substance. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. Δt is the change in the temperature. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and. What Makes A Good Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Makes A Good Calorimeter C is the specific heat of the body. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. Δt is the change in the temperature. calorimetry is used to measure amounts of heat transferred to. What Makes A Good Calorimeter.
From www.youtube.com
Constructing a Calorimeter YouTube What Makes A Good Calorimeter the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases. What Makes A Good Calorimeter.
From www.youtube.com
Learn about Calorimeter/ Calorimetry/Physics(Heat) 🔥 YouTube What Makes A Good Calorimeter The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases in temperature. Q is the measure of heat transfer. C is the specific heat of the body. — heat lost = heat gained. To do so, the heat is exchanged with a calibrated object. The heat transfer. What Makes A Good Calorimeter.
From exozspczc.blob.core.windows.net
Calorimeter Experiment Introduction at Jose Evans blog What Makes A Good Calorimeter some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. To do so, the heat is exchanged with a calibrated object. M is the mass of the body. Q is the measure of heat transfer. C is. What Makes A Good Calorimeter.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 What Makes A Good Calorimeter The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. M is the mass of the body. Δt is the change in the temperature. To do so, the heat is exchanged with a calibrated object. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. . What Makes A Good Calorimeter.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry What Makes A Good Calorimeter — heat lost = heat gained. Q is the measure of heat transfer. To do so, the heat is exchanged with a calibrated object. C is the specific heat of the body. some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. a calorimeter is a device used to measure the amount of heat. What Makes A Good Calorimeter.
From ambrosiabaking.com
Coffee Cup Calorimeter A Simple And Inexpensive Way To Measure The What Makes A Good Calorimeter calorimetry is used to measure amounts of heat transferred to or from a substance. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. C is the specific heat of. What Makes A Good Calorimeter.
From www.pinterest.com.au
Pin on Science Friday What Makes A Good Calorimeter a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. M is the mass of the body. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \). What Makes A Good Calorimeter.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Makes A Good Calorimeter Δt is the change in the temperature. M is the mass of the body. — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we have material of a uniform composition, we can relate the heat transferred from the hot object to the cold object by eq. calorimeter, device for measuring the. What Makes A Good Calorimeter.
From users.highland.edu
Calorimetry What Makes A Good Calorimeter The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. calorimetry is used to measure amounts of heat transferred to or from a substance. — heat lost = heat gained. Q is the measure of heat transfer. some of the most common types include reaction calorimeters, scanning calorimeters,. What Makes A Good Calorimeter.
From exoqfqtuz.blob.core.windows.net
Calorimetry Class 11 Questions at Vivian Higgins blog What Makes A Good Calorimeter — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we have material of a uniform composition, we can relate the heat transferred from the hot object to the cold object by eq. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. What Makes A Good Calorimeter.
From www.youtube.com
050 Calorimetry YouTube What Makes A Good Calorimeter The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases in temperature. — heat lost = heat gained. some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. M is the mass of the body. calorimetry is used to measure amounts of. What Makes A Good Calorimeter.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Makes A Good Calorimeter M is the mass of the body. calorimetry is used to measure amounts of heat transferred to or from a substance. C is the specific heat of the body. Q is the measure of heat transfer. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials.. What Makes A Good Calorimeter.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Makes A Good Calorimeter — heat lost = heat gained. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. To do so, the heat is exchanged with a calibrated object. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical. What Makes A Good Calorimeter.
From study.com
Calorimetry Definition, Equation & Types Lesson What Makes A Good Calorimeter calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Q is the measure of heat transfer. The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. The bomb calorimeter has an enclosure in which the reaction happens,. What Makes A Good Calorimeter.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube What Makes A Good Calorimeter The heat transfer in a system is calculated using the formula, \ (\begin {array} {l}q=mc\delta t\end {array} \) where. M is the mass of the body. The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases in temperature. calorimetry is used to measure amounts of heat transferred. What Makes A Good Calorimeter.
From eduinput.com
CalorimeterDefinition, History, Construction, Types, And Uses What Makes A Good Calorimeter To do so, the heat is exchanged with a calibrated object. Q is the measure of heat transfer. C is the specific heat of the body. The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases in temperature. — heat lost = heat gained. the most. What Makes A Good Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Makes A Good Calorimeter — heat lost = heat gained. Q is the measure of heat transfer. C is the specific heat of the body. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. a calorimeter is a device used to measure the amount of heat involved in. What Makes A Good Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry I What Makes A Good Calorimeter To do so, the heat is exchanged with a calibrated object. C is the specific heat of the body. — in an ideal calorimeter we consider the calorimeter to absorb no heat itself, so if we have material of a uniform composition, we can relate the heat transferred from the hot object to the cold object by eq. . What Makes A Good Calorimeter.
From scienceinfo.com
Calorimeter Definition, Types and Uses What Makes A Good Calorimeter Δt is the change in the temperature. the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. calorimetry is used to measure amounts of heat transferred to or from a substance. The bomb calorimeter has an enclosure in which the. What Makes A Good Calorimeter.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors What Makes A Good Calorimeter To do so, the heat is exchanged with a calibrated object. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. calorimetry is used to measure amounts of heat transferred to or from a substance. C is the specific heat of the body. — heat. What Makes A Good Calorimeter.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Makes A Good Calorimeter — heat lost = heat gained. The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases in temperature. some of the most common types include reaction calorimeters, scanning calorimeters, isothermal microcalorimeters,. Δt is the change in the temperature. To do so, the heat is exchanged with. What Makes A Good Calorimeter.
From ecampusontario.pressbooks.pub
5.2 Calorimetry Chemistry What Makes A Good Calorimeter The bomb calorimeter has an enclosure in which the reaction happens, surrounded by a liquid that absorbs the reaction’s heat and increases in temperature. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Q is the measure of heat transfer. — heat lost = heat gained. Δt is. What Makes A Good Calorimeter.
From byjus.com
What is bomb calorimeter? What Makes A Good Calorimeter the most common types of calorimeters are differential scanning calorimeters, titration calorimeters, isothermal micro. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Δt is the change in the temperature. C is the specific heat of the body. The bomb calorimeter has an enclosure in. What Makes A Good Calorimeter.