Iron Electrons In Each Shell . in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the initial two electrons in the electron configuration for iron will be in the 1s orbital. In the periodic table, the elements are listed in order of increasing atomic number z. For each electron shell atom. Electron configuration of iron is [ar] 3d6 4s2. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. iron atoms have 26 electrons and the shell structure is 2.8.14.2. The ground state electron configuration of ground state gaseous neutral iron is. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Because the 1s orbital can only hold two electrons, the next two. Possible oxidation states are +2,3.
from enginedbtersanctus.z22.web.core.windows.net
the initial two electrons in the electron configuration for iron will be in the 1s orbital. Electron configuration of iron is [ar] 3d6 4s2. For each electron shell atom. Because the 1s orbital can only hold two electrons, the next two. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Possible oxidation states are +2,3. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. In the periodic table, the elements are listed in order of increasing atomic number z. iron atoms have 26 electrons and the shell structure is 2.8.14.2. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there.
Iron Electron Config With Orbital Diagram
Iron Electrons In Each Shell Electron configuration of iron is [ar] 3d6 4s2. Because the 1s orbital can only hold two electrons, the next two. In the periodic table, the elements are listed in order of increasing atomic number z. For each electron shell atom. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Electron configuration of iron is [ar] 3d6 4s2. Possible oxidation states are +2,3. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. iron atoms have 26 electrons and the shell structure is 2.8.14.2. The ground state electron configuration of ground state gaseous neutral iron is. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior.
From www.nagwa.com
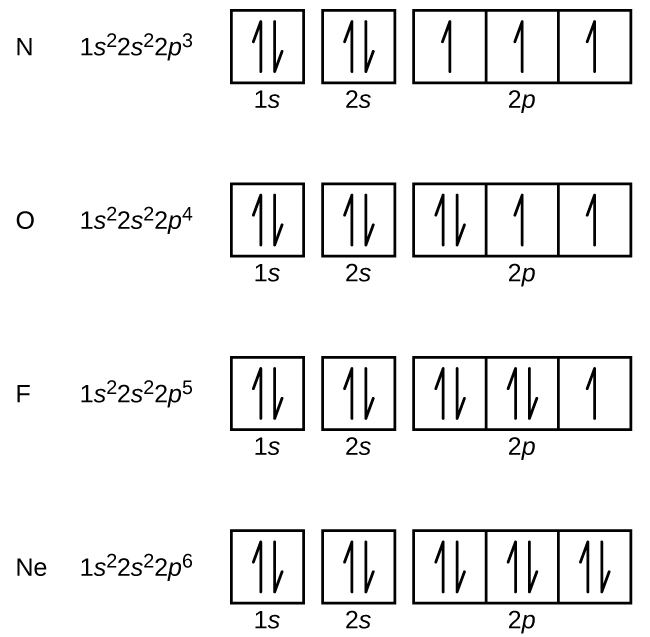
Question Video Finding the Number of Additional Electrons an Electron Iron Electrons In Each Shell the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Possible oxidation states are +2,3. iron atoms have 26 electrons and the shell structure is 2.8.14.2. Because. Iron Electrons In Each Shell.
From www.alamy.com
Fe Iron, Periodic Table of the Elements, Shell Structure of Iron Iron Electrons In Each Shell the initial two electrons in the electron configuration for iron will be in the 1s orbital. The ground state electron configuration of ground state gaseous neutral iron is. Because the 1s orbital can only hold two electrons, the next two. In the periodic table, the elements are listed in order of increasing atomic number z. Electron configuration of iron. Iron Electrons In Each Shell.
From www.slideserve.com
PPT Metals. Metalloids. Nonmetals. PowerPoint Presentation, free Iron Electrons In Each Shell Possible oxidation states are +2,3. For each electron shell atom. iron atoms have 26 electrons and the shell structure is 2.8.14.2. In the periodic table, the elements are listed in order of increasing atomic number z. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there.. Iron Electrons In Each Shell.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Iron Electrons In Each Shell the initial two electrons in the electron configuration for iron will be in the 1s orbital. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. For each electron shell atom. In the periodic table, the elements are listed in order of increasing atomic. Iron Electrons In Each Shell.
From enginedbtersanctus.z22.web.core.windows.net
Iron Electron Config With Orbital Diagram Iron Electrons In Each Shell Because the 1s orbital can only hold two electrons, the next two. Possible oxidation states are +2,3. Electron configuration of iron is [ar] 3d6 4s2. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. in order to write the iron electron configuration we first need to know the number of electrons for the. Iron Electrons In Each Shell.
From www.schoolmykids.com
Iron (Fe) Element Information, Facts, Properties, Uses Periodic Iron Electrons In Each Shell in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Because the 1s orbital can only hold two electrons, the next two. the initial two electrons in the electron configuration for iron will be in the 1s orbital. the number of electrons in each element’s. Iron Electrons In Each Shell.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Iron Electrons In Each Shell Because the 1s orbital can only hold two electrons, the next two. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. For each electron shell atom. Possible oxidation states are +2,3. Electron configuration of iron is [ar] 3d6 4s2. iron atoms have 26 electrons and. Iron Electrons In Each Shell.
From sciencenotes.org
Iron Atom Science Notes and Projects Iron Electrons In Each Shell iron atoms have 26 electrons and the shell structure is 2.8.14.2. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom. Because the 1s orbital can only hold two electrons, the next two. In the periodic table, the elements are listed in order of increasing atomic number z. Electron. Iron Electrons In Each Shell.
From exyczfhcx.blob.core.windows.net
Iron Electron Shell at Marcia Franklin blog Iron Electrons In Each Shell Because the 1s orbital can only hold two electrons, the next two. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. The ground state electron configuration of ground state gaseous neutral iron is. iron atoms have 26 electrons and the shell structure is 2.8.14.2. Electron configuration of iron is [ar] 3d6 4s2. In. Iron Electrons In Each Shell.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Iron Electrons In Each Shell In the periodic table, the elements are listed in order of increasing atomic number z. Possible oxidation states are +2,3. iron atoms have 26 electrons and the shell structure is 2.8.14.2. For each electron shell atom. Electron configuration of iron is [ar] 3d6 4s2. Because the 1s orbital can only hold two electrons, the next two. the number. Iron Electrons In Each Shell.
From exyczfhcx.blob.core.windows.net
Iron Electron Shell at Marcia Franklin blog Iron Electrons In Each Shell here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom. Because the 1s orbital can only hold two electrons, the next two. Electron configuration of iron is [ar] 3d6 4s2. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in. Iron Electrons In Each Shell.
From www.bigstockphoto.com
Iron. Atom Structure Vector & Photo Bigstock Iron Electrons In Each Shell the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. the initial two electrons in the electron configuration for iron will be in the 1s orbital. In the periodic. Iron Electrons In Each Shell.
From ceaulups.blob.core.windows.net
Iron Has How Many Electrons In The Shells at Nicole Paige blog Iron Electrons In Each Shell Possible oxidation states are +2,3. Because the 1s orbital can only hold two electrons, the next two. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. For each electron. Iron Electrons In Each Shell.
From www.webelements.com
Elements Periodic Table » Iron » properties of free atoms Iron Electrons In Each Shell Electron configuration of iron is [ar] 3d6 4s2. Possible oxidation states are +2,3. iron atoms have 26 electrons and the shell structure is 2.8.14.2. Because the 1s orbital can only hold two electrons, the next two. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom. In the periodic. Iron Electrons In Each Shell.
From mavink.com
Electron Orbital Configuration Chart Iron Electrons In Each Shell Because the 1s orbital can only hold two electrons, the next two. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. Electron configuration of iron is [ar] 3d6 4s2. For each electron shell atom. in order to write the iron electron configuration we first need to know the number of electrons for the. Iron Electrons In Each Shell.
From www.nagwa.com
Question Video Identifying the Maximum Number of Electrons in the 1st Iron Electrons In Each Shell The ground state electron configuration of ground state gaseous neutral iron is. iron atoms have 26 electrons and the shell structure is 2.8.14.2. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. For each electron shell atom. the initial two electrons in. Iron Electrons In Each Shell.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table Iron Electrons In Each Shell Possible oxidation states are +2,3. the initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, the next two. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. In the periodic. Iron Electrons In Each Shell.
From www.verticallearning.org
Grade 7 Vertical Science Iron Electrons In Each Shell In the periodic table, the elements are listed in order of increasing atomic number z. The ground state electron configuration of ground state gaseous neutral iron is. iron atoms have 26 electrons and the shell structure is 2.8.14.2. Electron configuration of iron is [ar] 3d6 4s2. the number of electrons in each element’s electron shells, particularly the outermost. Iron Electrons In Each Shell.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Electrons In Each Shell iron atoms have 26 electrons and the shell structure is 2.8.14.2. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. the initial two electrons in the electron configuration for iron will be in the 1s orbital. the number of electrons in each element’s. Iron Electrons In Each Shell.
From ceaulups.blob.core.windows.net
Iron Has How Many Electrons In The Shells at Nicole Paige blog Iron Electrons In Each Shell Because the 1s orbital can only hold two electrons, the next two. Electron configuration of iron is [ar] 3d6 4s2. In the periodic table, the elements are listed in order of increasing atomic number z. Possible oxidation states are +2,3. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in. Iron Electrons In Each Shell.
From www.scienceabc.com
Octet Rule Why Are Atoms With 8 Valence Electrons So Stable? Iron Electrons In Each Shell Electron configuration of iron is [ar] 3d6 4s2. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. the initial two electrons in the electron configuration for iron will be in the 1s orbital. in order to write the iron electron configuration we first need to know the number of electrons for the. Iron Electrons In Each Shell.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Electrons In Each Shell Because the 1s orbital can only hold two electrons, the next two. In the periodic table, the elements are listed in order of increasing atomic number z. Possible oxidation states are +2,3. iron atoms have 26 electrons and the shell structure is 2.8.14.2. Electron configuration of iron is [ar] 3d6 4s2. here are electron shell atom diagrams for. Iron Electrons In Each Shell.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Electrons In Each Shell here are electron shell atom diagrams for the elements, ordered by increasing atomic number. iron atoms have 26 electrons and the shell structure is 2.8.14.2. Electron configuration of iron is [ar] 3d6 4s2. For each electron shell atom. The ground state electron configuration of ground state gaseous neutral iron is. the number of electrons in each element’s. Iron Electrons In Each Shell.
From imgbin.com
Valence Electron Electron Shell Electron Configuration Chemical Element Iron Electrons In Each Shell Electron configuration of iron is [ar] 3d6 4s2. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. In the periodic table, the elements are listed in order of increasing atomic number z. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its. Iron Electrons In Each Shell.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Iron Electrons In Each Shell The ground state electron configuration of ground state gaseous neutral iron is. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. In the periodic table, the elements are listed in order of increasing atomic number z. iron atoms have 26 electrons and the shell structure. Iron Electrons In Each Shell.
From ar.inspiredpencil.com
Electron Configuration Of Iron Iron Electrons In Each Shell the initial two electrons in the electron configuration for iron will be in the 1s orbital. Because the 1s orbital can only hold two electrons, the next two. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Possible oxidation states are +2,3. here are. Iron Electrons In Each Shell.
From exyhjhprp.blob.core.windows.net
Iron Electron Shell Configuration at Karen Clifford blog Iron Electrons In Each Shell Possible oxidation states are +2,3. In the periodic table, the elements are listed in order of increasing atomic number z. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. The ground state electron configuration of ground state gaseous neutral iron is. the initial two electrons in the electron configuration for iron will be. Iron Electrons In Each Shell.
From spmkimia.onlinetuition.com.my
Susunan Elektron di Dalam Atom Nota Ulangkaji Kimia SPM Tingkatan 4 Iron Electrons In Each Shell The ground state electron configuration of ground state gaseous neutral iron is. the initial two electrons in the electron configuration for iron will be in the 1s orbital. here are electron shell atom diagrams for the elements, ordered by increasing atomic number. For each electron shell atom. Electron configuration of iron is [ar] 3d6 4s2. In the periodic. Iron Electrons In Each Shell.
From mungfali.com
How Many Electrons Are In Each Shell Iron Electrons In Each Shell iron atoms have 26 electrons and the shell structure is 2.8.14.2. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. In the periodic table, the elements are listed in order of increasing atomic number z. the initial two electrons in the electron. Iron Electrons In Each Shell.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Electrons In Each Shell Because the 1s orbital can only hold two electrons, the next two. For each electron shell atom. Electron configuration of iron is [ar] 3d6 4s2. iron atoms have 26 electrons and the shell structure is 2.8.14.2. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical. Iron Electrons In Each Shell.
From icdsc.org
How many electrons are in each shell including 3p orbitals Iron Electrons In Each Shell For each electron shell atom. The ground state electron configuration of ground state gaseous neutral iron is. Possible oxidation states are +2,3. iron atoms have 26 electrons and the shell structure is 2.8.14.2. Electron configuration of iron is [ar] 3d6 4s2. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary. Iron Electrons In Each Shell.
From opentextbc.ca
6.4 Electronic Structure of Atoms (Electron Configurations) Chemistry Iron Electrons In Each Shell In the periodic table, the elements are listed in order of increasing atomic number z. iron atoms have 26 electrons and the shell structure is 2.8.14.2. The ground state electron configuration of ground state gaseous neutral iron is. the initial two electrons in the electron configuration for iron will be in the 1s orbital. here are electron. Iron Electrons In Each Shell.
From exyikuzef.blob.core.windows.net
What Is The Electron Shell Arrangement For Iron at Lynn Olson blog Iron Electrons In Each Shell Electron configuration of iron is [ar] 3d6 4s2. In the periodic table, the elements are listed in order of increasing atomic number z. in order to write the iron electron configuration we first need to know the number of electrons for the fe atom (there. Possible oxidation states are +2,3. the initial two electrons in the electron configuration. Iron Electrons In Each Shell.
From material-properties.org
Iron Periodic Table and Atomic Properties Iron Electrons In Each Shell Because the 1s orbital can only hold two electrons, the next two. In the periodic table, the elements are listed in order of increasing atomic number z. iron atoms have 26 electrons and the shell structure is 2.8.14.2. in order to write the iron electron configuration we first need to know the number of electrons for the fe. Iron Electrons In Each Shell.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Electrons In Each Shell For each electron shell atom. the number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. Because the 1s orbital can only hold two electrons, the next two. in order to write the iron electron configuration we first need to know the number of electrons. Iron Electrons In Each Shell.