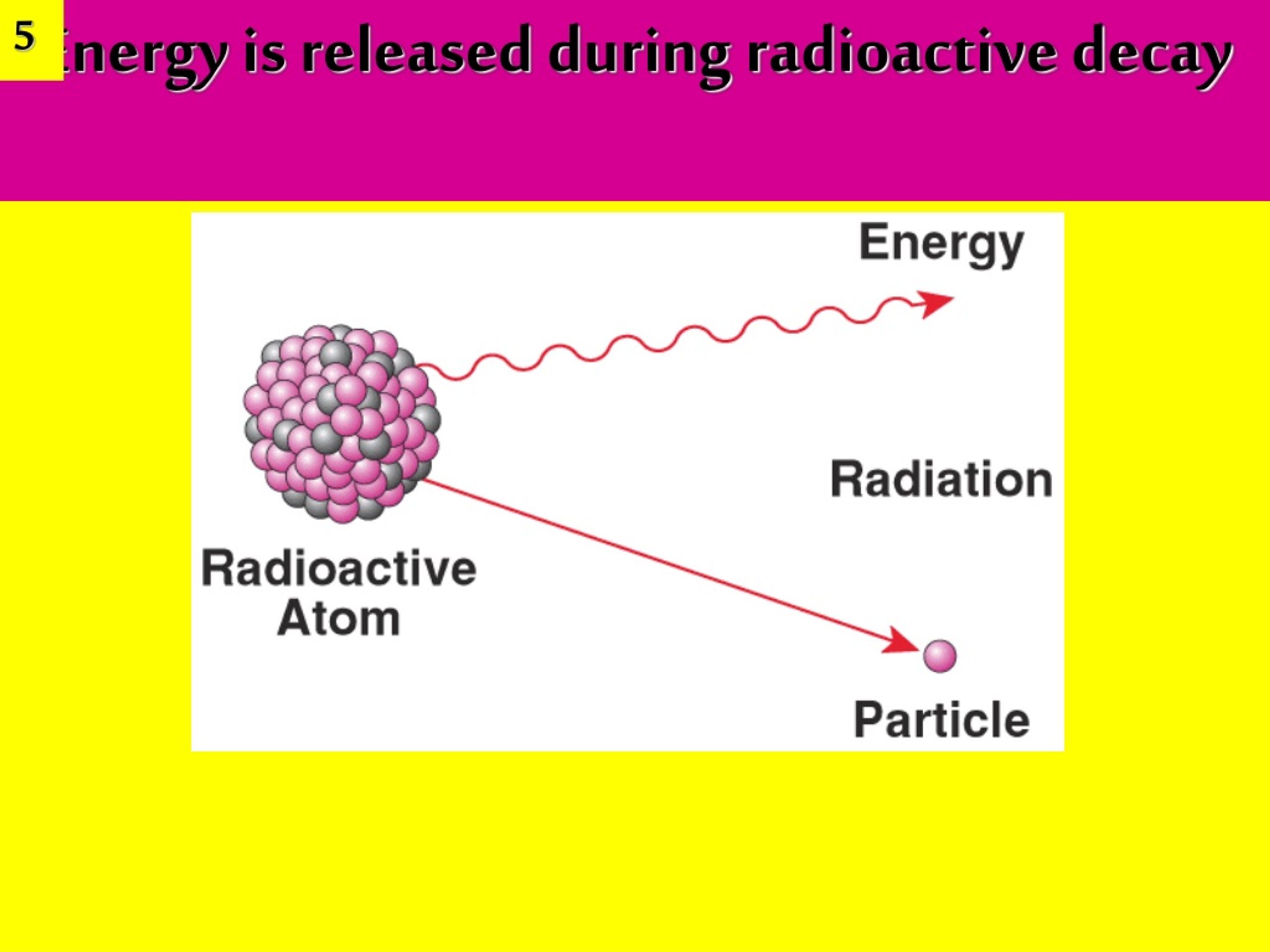
Radioactive Decay Simple Definition Chemistry . The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. the spontaneous change of an unstable nuclide into another is radioactive decay. radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. when an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. Classify a radioactive decay as a combination or a decomposition reaction. Identify the indicator word associated with. It is, in essence, an. recognize common modes of radioactive decay; The unstable nuclide is called the parent nuclide; The daughter nuclide may be stable, or it may decay itself. To reach a lower, more stable energy level, it releases energy in the form of radiation. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. Identify common particles and energies involved in nuclear decay.
from www.slideserve.com
radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. when an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. The nuclide that results from the decay is known as the daughter nuclide. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. recognize common modes of radioactive decay; The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. the spontaneous change of an unstable nuclide into another is radioactive decay. Identify the indicator word associated with. The daughter nuclide may be stable, or it may decay itself. It is, in essence, an.
PPT Radioactivity PowerPoint Presentation, free download ID296273
Radioactive Decay Simple Definition Chemistry To reach a lower, more stable energy level, it releases energy in the form of radiation. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. To reach a lower, more stable energy level, it releases energy in the form of radiation. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. recognize common modes of radioactive decay; radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. when an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. The unstable nuclide is called the parent nuclide; the spontaneous change of an unstable nuclide into another is radioactive decay. The daughter nuclide may be stable, or it may decay itself. Classify a radioactive decay as a combination or a decomposition reaction. The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Identify common particles and energies involved in nuclear decay. Identify the indicator word associated with.
From www.alamy.com
Radioactive decay diagram hires stock photography and images Alamy Radioactive Decay Simple Definition Chemistry radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. Identify common particles and energies involved in nuclear decay. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. The nuclide that results from the decay is known as the daughter nuclide.. Radioactive Decay Simple Definition Chemistry.
From studiousguy.com
7 Radioactive Decay Examples in Real Life StudiousGuy Radioactive Decay Simple Definition Chemistry It is, in essence, an. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. The unstable nuclide is called the parent nuclide; radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. The nuclide that results from the decay is known as the daughter nuclide. To reach. Radioactive Decay Simple Definition Chemistry.
From www.scienceabc.com
Why Are Certain Elements Radioactive? » ScienceABC Radioactive Decay Simple Definition Chemistry This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. Identify common particles and energies involved in nuclear decay. The nuclide that results from the decay is known as the daughter nuclide. The daughter nuclide. Radioactive Decay Simple Definition Chemistry.
From animalia-life.club
Alpha Beta Gamma Decay Radioactive Decay Simple Definition Chemistry radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. The nuclide that results from the decay is known as the daughter nuclide. Classify a radioactive decay as a combination or a decomposition reaction.. Radioactive Decay Simple Definition Chemistry.
From www.vrogue.co
What Is Radioactive Decay Definition vrogue.co Radioactive Decay Simple Definition Chemistry The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. Identify common particles and. Radioactive Decay Simple Definition Chemistry.
From study.com
Writing Typical Radioactive Decay Equations Chemistry Radioactive Decay Simple Definition Chemistry Identify the indicator word associated with. the spontaneous change of an unstable nuclide into another is radioactive decay. when an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. recognize common modes of radioactive decay; radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. . Radioactive Decay Simple Definition Chemistry.
From www.sliderbase.com
Radioactive Decay, Nuclear Reactions Presentation Physics Radioactive Decay Simple Definition Chemistry The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. The unstable nuclide is called the parent nuclide; Identify the indicator word associated with. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. radioactivity is the spontaneous emission. Radioactive Decay Simple Definition Chemistry.
From mavink.com
4 Types Of Radioactive Decay Radioactive Decay Simple Definition Chemistry Identify common particles and energies involved in nuclear decay. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. the spontaneous change of an unstable nuclide into another is radioactive decay.. Radioactive Decay Simple Definition Chemistry.
From www.env.go.jp
Halflives and Radioactive Decay [MOE] Radioactive Decay Simple Definition Chemistry The nuclide that results from the decay is known as the daughter nuclide. The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. Identify common particles and energies involved in nuclear decay. radioactivity, property exhibited. Radioactive Decay Simple Definition Chemistry.
From chem.libretexts.org
15.2 HalfLife Chemistry LibreTexts Radioactive Decay Simple Definition Chemistry The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. the spontaneous change of an unstable nuclide into another is radioactive decay. The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. This radiation can be emitted as particles or electromagnetic. Radioactive Decay Simple Definition Chemistry.
From study.com
Alpha Decay Equation, Formula, & Reaction Radioactive Decay Simple Definition Chemistry The unstable nuclide is called the parent nuclide; Classify a radioactive decay as a combination or a decomposition reaction. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. It is, in essence, an. Identify common particles and energies involved in nuclear decay. radioactivity, property exhibited by certain types of matter of emitting energy and. Radioactive Decay Simple Definition Chemistry.
From sciencenotes.org
Radioactivity and the Types of Radioactive Decay Radioactive Decay Simple Definition Chemistry radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. when an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. The daughter nuclide may be stable, or. Radioactive Decay Simple Definition Chemistry.
From www.tessshebaylo.com
Uranium Radioactive Decay Equation Tessshebaylo Radioactive Decay Simple Definition Chemistry recognize common modes of radioactive decay; Identify the indicator word associated with. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. The daughter nuclide may be stable, or it may decay itself. radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. To reach. Radioactive Decay Simple Definition Chemistry.
From ausearthed.blogspot.com
Radioactive Popcorn Radioactive Decay Simple Definition Chemistry This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. The daughter nuclide may be stable, or it may decay itself. Identify the indicator word associated with. To reach a lower, more stable energy level, it releases energy in. Radioactive Decay Simple Definition Chemistry.
From livingroom2021.blogspot.com
Radioactive Decay Simple Definition What is radioactivity? Quora Radioactive Decay Simple Definition Chemistry It is, in essence, an. Identify the indicator word associated with. the spontaneous change of an unstable nuclide into another is radioactive decay. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. recognize common modes of radioactive decay; Radioactive decay occurs for all nuclei with. Radioactive Decay Simple Definition Chemistry.
From www.slideserve.com
PPT Radioactivity PowerPoint Presentation, free download ID296273 Radioactive Decay Simple Definition Chemistry The daughter nuclide may be stable, or it may decay itself. The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. To reach a lower, more stable energy level, it releases energy in the form of radiation. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other. Radioactive Decay Simple Definition Chemistry.
From studiousguy.com
7 Radioactive Decay Examples in Real Life StudiousGuy Radioactive Decay Simple Definition Chemistry radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Classify a radioactive decay as a combination or a decomposition reaction. recognize. Radioactive Decay Simple Definition Chemistry.
From www.radiation-dosimetry.org
What is Radioactive Decay Definition Radioactive Decay Simple Definition Chemistry This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Identify common particles and energies involved in nuclear decay. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. Identify the indicator word associated with. radioactivity, property exhibited by certain types of matter of. Radioactive Decay Simple Definition Chemistry.
From www.scienceabc.com
Why Is The Term "HalfLife" Used To Measure Radioactivity? » ScienceABC Radioactive Decay Simple Definition Chemistry The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. It is, in essence, an. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of. Radioactive Decay Simple Definition Chemistry.
From www.snexplores.org
Explainer Radiation and radioactive decay Radioactive Decay Simple Definition Chemistry radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. The decay rate is. Radioactive Decay Simple Definition Chemistry.
From www.slideserve.com
PPT Radioactive Decay PowerPoint Presentation, free download ID83468 Radioactive Decay Simple Definition Chemistry Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. recognize common modes of radioactive decay; The. Radioactive Decay Simple Definition Chemistry.
From studylib.net
Radioactive Decay Radioactive Decay Simple Definition Chemistry To reach a lower, more stable energy level, it releases energy in the form of radiation. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. It is, in essence, an. the spontaneous change of an unstable nuclide into another is radioactive decay. radioactivity is the spontaneous emission of ionizing. Radioactive Decay Simple Definition Chemistry.
From www.scienceabc.com
Why Are Certain Elements Radioactive? » ScienceABC Radioactive Decay Simple Definition Chemistry The unstable nuclide is called the parent nuclide; Identify the indicator word associated with. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. The three main types of radioactive decay are alpha, beta, and gamma decay, but there are other nuclear reactions responsible for radioactivity. radioactivity, property exhibited by certain. Radioactive Decay Simple Definition Chemistry.
From www.shutterstock.com
Radioactive Decay Atom Showing Energy Particle 库存矢量图(免版税)1170935842 Radioactive Decay Simple Definition Chemistry Identify the indicator word associated with. The daughter nuclide may be stable, or it may decay itself. This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. radioactivity, property exhibited by certain types of. Radioactive Decay Simple Definition Chemistry.
From www.youtube.com
Radioactivity (11 of 16) Radioactive Decay Law, An Explanation YouTube Radioactive Decay Simple Definition Chemistry radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. Classify a radioactive decay as a combination or a decomposition. Radioactive Decay Simple Definition Chemistry.
From mavink.com
4 Types Of Radioactive Decay Radioactive Decay Simple Definition Chemistry The unstable nuclide is called the parent nuclide; The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. the spontaneous change of an unstable nuclide into another is radioactive decay. It is, in essence, an. radioactivity is the spontaneous emission of ionizing radiation from nuclear decay and reactions. Identify the indicator word. Radioactive Decay Simple Definition Chemistry.
From www.tessshebaylo.com
Radioactive Decay Equation Chemistry Tessshebaylo Radioactive Decay Simple Definition Chemistry the spontaneous change of an unstable nuclide into another is radioactive decay. The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. Identify the indicator word associated with. when an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. The daughter nuclide may be stable,. Radioactive Decay Simple Definition Chemistry.
From www.youtube.com
Nuclear Chemistry & Radioactive Decay Practice Problems YouTube Radioactive Decay Simple Definition Chemistry The unstable nuclide is called the parent nuclide; Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. recognize common modes of radioactive decay; The decay rate is proportional to the number of original (undecayed) nuclei n in a substance. Identify common particles and energies involved in nuclear decay. It is,. Radioactive Decay Simple Definition Chemistry.
From www.epa.gov
Radioactive Decay Radiation Protection US EPA Radioactive Decay Simple Definition Chemistry Classify a radioactive decay as a combination or a decomposition reaction. recognize common modes of radioactive decay; It is, in essence, an. Identify the indicator word associated with. To reach a lower, more stable energy level, it releases energy in the form of radiation. radioactivity occurs when an atom has an excess of energy, mass, or both, making. Radioactive Decay Simple Definition Chemistry.
From www.varsitytutors.com
Help with Radioactive Decay High School Chemistry Radioactive Decay Simple Definition Chemistry It is, in essence, an. Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. Classify a radioactive decay as a combination or a decomposition reaction. Identify the indicator word associated with. Identify common particles and energies involved in nuclear decay. The three main types of radioactive decay are alpha, beta, and. Radioactive Decay Simple Definition Chemistry.
From www.pinterest.com
Changes of N and Z caused by radioactive decay and representation in Radioactive Decay Simple Definition Chemistry recognize common modes of radioactive decay; To reach a lower, more stable energy level, it releases energy in the form of radiation. radioactivity occurs when an atom has an excess of energy, mass, or both, making its nucleus unstable. the spontaneous change of an unstable nuclide into another is radioactive decay. radioactivity is the spontaneous emission. Radioactive Decay Simple Definition Chemistry.
From www.slideserve.com
PPT 4. Radioactive Decay PowerPoint Presentation ID243508 Radioactive Decay Simple Definition Chemistry radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. The unstable nuclide is called the parent nuclide; This radiation can be emitted as particles or electromagnetic waves, depending on the nature of the decay. Classify a radioactive decay as a combination or a decomposition reaction. recognize common modes of radioactive decay; . Radioactive Decay Simple Definition Chemistry.
From www.slideserve.com
PPT Radioactive Decay PowerPoint Presentation, free download ID1904215 Radioactive Decay Simple Definition Chemistry Classify a radioactive decay as a combination or a decomposition reaction. when an individual nucleus transforms into another with the emission of radiation, the nucleus is said to decay. The daughter nuclide may be stable, or it may decay itself. recognize common modes of radioactive decay; This radiation can be emitted as particles or electromagnetic waves, depending on. Radioactive Decay Simple Definition Chemistry.
From studylib.net
Radioactive Decay Radioactive Decay Simple Definition Chemistry It is, in essence, an. Identify common particles and energies involved in nuclear decay. the spontaneous change of an unstable nuclide into another is radioactive decay. The daughter nuclide may be stable, or it may decay itself. The unstable nuclide is called the parent nuclide; To reach a lower, more stable energy level, it releases energy in the form. Radioactive Decay Simple Definition Chemistry.
From www.vrogue.co
Law Of Radioactive Decay vrogue.co Radioactive Decay Simple Definition Chemistry Radioactive decay occurs for all nuclei with z> 82, and also for some unstable isotopes with z <83. radioactivity, property exhibited by certain types of matter of emitting energy and subatomic particles spontaneously. The nuclide that results from the decay is known as the daughter nuclide. The three main types of radioactive decay are alpha, beta, and gamma decay,. Radioactive Decay Simple Definition Chemistry.