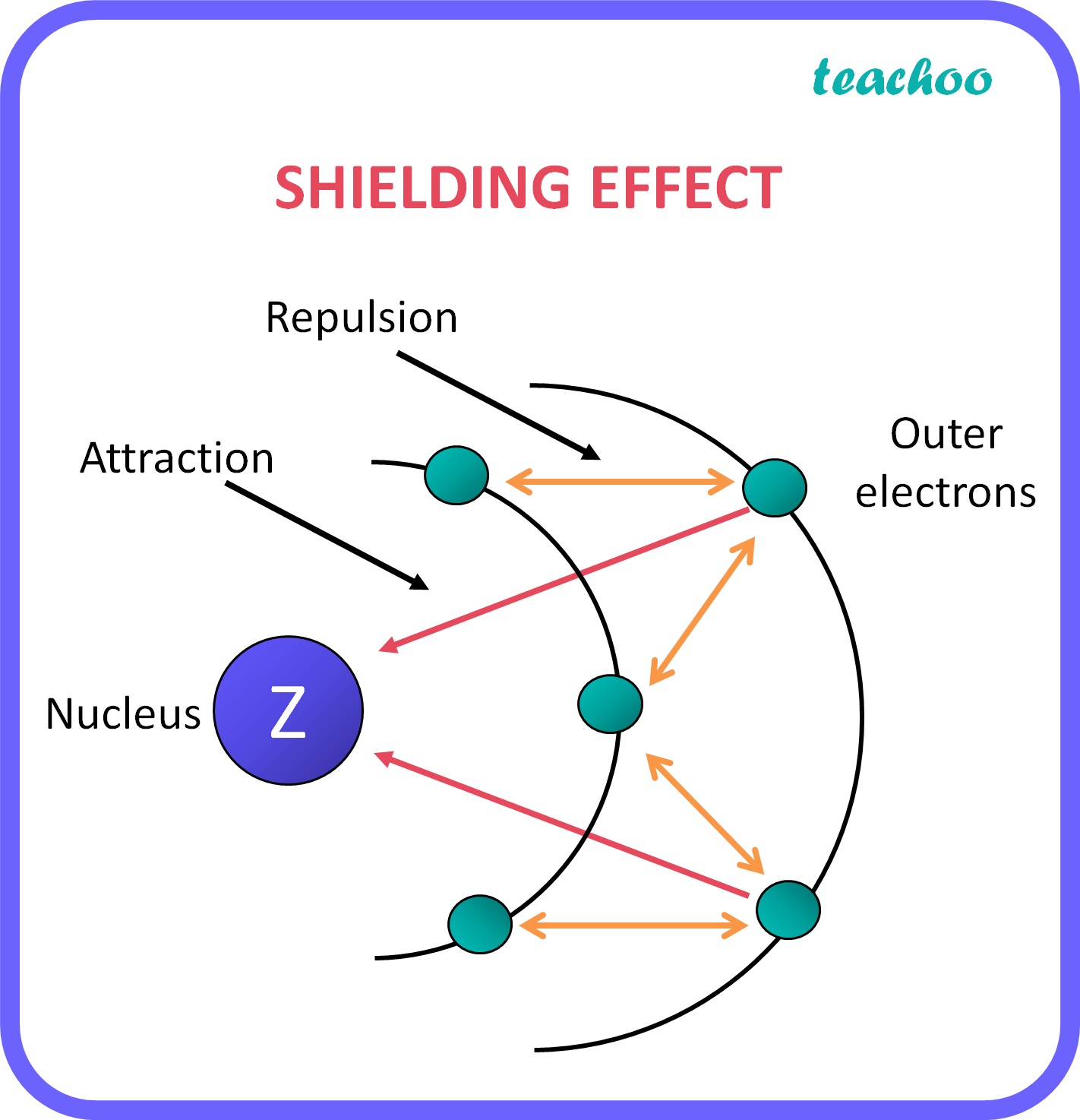
What Is A Shielding Electron . Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). — what is shielding effect? the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use.
from exoeftfzx.blob.core.windows.net
At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. — what is shielding effect? the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.
What Is Shielding Chem at Victor Riggs blog
What Is A Shielding Electron The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). — what is shielding effect? the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free What Is A Shielding Electron — what is shielding effect? the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. Electrons in an \(s\) orbital can shield \(p\) electrons at the same. What Is A Shielding Electron.
From slidetodoc.com
The Periodic Table Chapter 4 4 1 Mendeleevs What Is A Shielding Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. — what is shielding effect? At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). Electrons in an \(s\). What Is A Shielding Electron.
From educationfruits.z21.web.core.windows.net
What Is Shielding Effect In Chemistry What Is A Shielding Electron At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an atom contains protons, which carry positive. What Is A Shielding Electron.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick What Is A Shielding Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. — what is shielding effect? At the same time, these valence electrons are also repelled by the. What Is A Shielding Electron.
From www.nuclear-power.com
Shielding of Neutron Radiation Types & Uses What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. — what is shielding effect? the concept of electron shielding, in which intervening electrons act to reduce. What Is A Shielding Electron.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these. What Is A Shielding Electron.
From socratic.org
How are shielding effect and atomic radius related? Socratic What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. — what is shielding effect? The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. the concept of electron shielding, in which intervening electrons act to reduce. What Is A Shielding Electron.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive What Is A Shielding Electron The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. What Is A Shielding Electron.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog What Is A Shielding Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. — what is shielding effect? At the same time, these valence electrons. What Is A Shielding Electron.
From www.slideserve.com
PPT Chapter 6 Periodic Table PowerPoint Presentation, free download What Is A Shielding Electron — what is shielding effect? the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. Electrons in an \(s\) orbital can shield \(p\) electrons at the same. What Is A Shielding Electron.
From www.slideserve.com
PPT Periodic Table Chapter PowerPoint Presentation, free download What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). the concept of electron shielding, in which intervening electrons act. What Is A Shielding Electron.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity What Is A Shielding Electron — what is shielding effect? At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an. What Is A Shielding Electron.
From byjus.com
What is shielding and deshielding in NMR? Give an example? What Is A Shielding Electron The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. — what is shielding effect? At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). Electrons in an \(s\) orbital can shield \(p\) electrons at. What Is A Shielding Electron.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID1951146 What Is A Shielding Electron At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). — what is shielding effect? the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an. What Is A Shielding Electron.
From ar.inspiredpencil.com
Shielding Effect Electrons What Is A Shielding Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). Electrons in an \(s\) orbital can shield \(p\) electrons at. What Is A Shielding Electron.
From www.youtube.com
Orbital shielding and YouTube What Is A Shielding Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. — what is shielding effect? Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. At the same time, these valence electrons. What Is A Shielding Electron.
From www.slideserve.com
PPT Chapter 8 Periodic Properties & Electron Configurations Bushra What Is A Shielding Electron The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. — what is shielding effect? At the same time, these valence electrons are also repelled by the. What Is A Shielding Electron.
From chem.libretexts.org
3.2 Shielding Chemistry LibreTexts What Is A Shielding Electron At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. — what is shielding effect? The nucleus of an atom. What Is A Shielding Electron.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. — what is shielding effect? At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). The nucleus of an atom. What Is A Shielding Electron.
From general.chemistrysteps.com
Ionization energy Chemistry Steps What Is A Shielding Electron The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. At the same time, these valence electrons are also repelled by the electrons present in the inner shells. What Is A Shielding Electron.
From www.slideserve.com
PPT Chapter 6 The Periodic Table PowerPoint Presentation, free What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. — what is shielding effect? At the same time, these valence electrons are also repelled by the electrons. What Is A Shielding Electron.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID5583827 What Is A Shielding Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical. What Is A Shielding Electron.
From ar.inspiredpencil.com
Electron Shielding What Is A Shielding Electron At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of. What Is A Shielding Electron.
From www.theengineeringprojects.com
Periodic Table of Elements Definition, Groups & Trends The What Is A Shielding Electron — what is shielding effect? The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. At the same time, these valence electrons are also repelled by the. What Is A Shielding Electron.
From www.slideserve.com
PPT Unit 5 The Periodic Table PowerPoint Presentation, free download What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. What Is A Shielding Electron.
From www.breakingatom.com
Shielding of the Nucleus What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by. What Is A Shielding Electron.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. At the same time, these valence electrons are also repelled by the electrons. What Is A Shielding Electron.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube What Is A Shielding Electron — what is shielding effect? At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an. What Is A Shielding Electron.
From exoeftfzx.blob.core.windows.net
What Is Shielding Chem at Victor Riggs blog What Is A Shielding Electron — what is shielding effect? Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). The nucleus of an atom. What Is A Shielding Electron.
From localrevive.com
LearnEMC Introduction to Practical Shielding (2022) What Is A Shielding Electron — what is shielding effect? Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. the concept of electron shielding, in which intervening electrons act to reduce. What Is A Shielding Electron.
From wisc.pb.unizin.org
M7Q8 Core and Valence Electrons, Shielding, Zeff Chem 103/104 What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an atom contains protons, which carry positive charges, while electrons. What Is A Shielding Electron.
From www.youtube.com
Electron shielding YouTube What Is A Shielding Electron At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). — what is shielding effect? The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. Electrons in an \(s\) orbital can shield \(p\) electrons at. What Is A Shielding Electron.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID2836425 What Is A Shielding Electron — what is shielding effect? The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. At the same time, these valence electrons are also repelled by the electrons present in the inner shells (these inner electrons are also called intervening electrons). Electrons in an \(s\) orbital can shield \(p\) electrons at. What Is A Shielding Electron.
From www.slideserve.com
PPT Chapter 6 Chemical Bonding PowerPoint Presentation, free What Is A Shielding Electron Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital. the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. The nucleus of an atom contains protons, which carry positive charges, while electrons. What Is A Shielding Electron.
From www.breakingatom.com
Shielding What Is A Shielding Electron the concept of electron shielding, in which intervening electrons act to reduce the positive nuclear charge experienced by an electron, allows the use. — what is shielding effect? The nucleus of an atom contains protons, which carry positive charges, while electrons orbit around the nucleus in. Electrons in an \(s\) orbital can shield \(p\) electrons at the same. What Is A Shielding Electron.