Equivalence Point In Coulometric Titration . The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Coulometric titrations are in many ways similar to volumetric titrations: An overview of possible titrations is given in table 1 [4]. Metric titrations can be performed. The equivalence point or end point of a coulometric. Strong and weak acids and bases. An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant.
from app.jove.com
The equivalence point or end point of a coulometric. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. Coulometric titrations are in many ways similar to volumetric titrations: Strong and weak acids and bases. Metric titrations can be performed. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. An overview of possible titrations is given in table 1 [4].
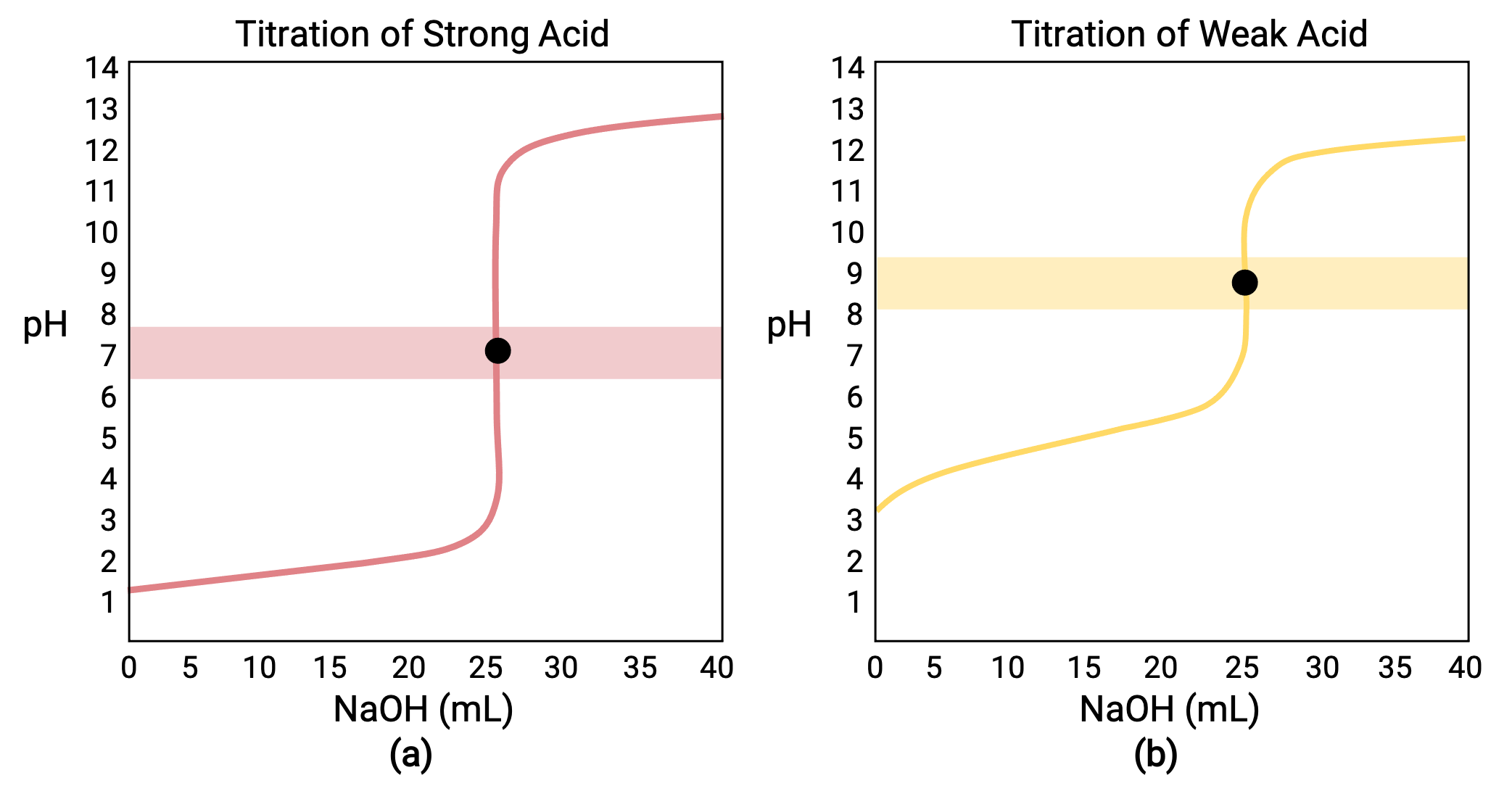
AcidBase/ pH Titration Curves and Equivalence Points Concept
Equivalence Point In Coulometric Titration The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Metric titrations can be performed. An overview of possible titrations is given in table 1 [4]. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. The equivalence point or end point of a coulometric. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Strong and weak acids and bases. Coulometric titrations are in many ways similar to volumetric titrations:
From www.researchgate.net
Coulometric titration curve for a LiSi system at 450 • C (dotted Equivalence Point In Coulometric Titration Metric titrations can be performed. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Coulometric titrations are in many ways similar to volumetric titrations: Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. Let us consider the oxidation of fe2+ to fe3+. Equivalence Point In Coulometric Titration.
From www.researchgate.net
Coulometricpotentiometric titration curve of THAM in aqueous sodium Equivalence Point In Coulometric Titration The equivalence point or end point of a coulometric. Coulometric titrations are in many ways similar to volumetric titrations: Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. The present paper is a. Equivalence Point In Coulometric Titration.
From klaakyfwn.blob.core.windows.net
Dynamic Equivalence Point Titration at Sonja Hogan blog Equivalence Point In Coulometric Titration The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Strong and weak acids and bases. Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration.. Equivalence Point In Coulometric Titration.
From loeigruoo.blob.core.windows.net
How To Do A Titration Graph at Terry Bailey blog Equivalence Point In Coulometric Titration Metric titrations can be performed. An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Let. Equivalence Point In Coulometric Titration.
From studylib.net
b.) Advantages of Coulometry Equivalence Point In Coulometric Titration An overview of possible titrations is given in table 1 [4]. Coulometric titrations are in many ways similar to volumetric titrations: Strong and weak acids and bases. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. Consider the reaction below in which fe 2 + is the. Equivalence Point In Coulometric Titration.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Equivalence Point In Coulometric Titration Coulometric titrations are in many ways similar to volumetric titrations: Metric titrations can be performed. An overview of possible titrations is given in table 1 [4]. The equivalence point or end point of a coulometric. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. Consider the reaction. Equivalence Point In Coulometric Titration.
From dxouckyfl.blob.core.windows.net
Coulometric Karl Fischer Titration Pdf at Brian Malm blog Equivalence Point In Coulometric Titration Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. Coulometric titrations are in many ways similar to volumetric titrations: An overview of possible titrations is given in table 1 [4]. An examination of the two standard potentials for the two half reactions confirms that in this pair. Equivalence Point In Coulometric Titration.
From www.bartleby.com
Answered Ca(OH)2 Titration Data for Ksp 12… bartleby Equivalence Point In Coulometric Titration Metric titrations can be performed. The equivalence point or end point of a coulometric. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Coulometric titrations are in many ways similar to volumetric titrations: Strong and weak acids and bases. Consider the reaction below in which fe 2 + is the analyte. Equivalence Point In Coulometric Titration.
From exyjcpegf.blob.core.windows.net
Titration And Equivalence Point at Bill Hubbard blog Equivalence Point In Coulometric Titration An overview of possible titrations is given in table 1 [4]. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. An examination of the two standard potentials for. Equivalence Point In Coulometric Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Equivalence Point In Coulometric Titration Coulometric titrations are in many ways similar to volumetric titrations: The equivalence point or end point of a coulometric. An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. Metric titrations can be performed. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration.. Equivalence Point In Coulometric Titration.
From www.reddit.com
[Grade 11 chemistry titration] Titratiok curve with two equivalence Equivalence Point In Coulometric Titration Coulometric titrations are in many ways similar to volumetric titrations: Metric titrations can be performed. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. An examination of the two standard potentials for. Equivalence Point In Coulometric Titration.
From www.studypool.com
SOLUTION Coulometric titration procedure Studypool Equivalence Point In Coulometric Titration The equivalence point or end point of a coulometric. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. An overview of possible titrations is given in table 1. Equivalence Point In Coulometric Titration.
From sciencetech.cz
Coulometric Titrator Sciencetech Equivalence Point In Coulometric Titration Metric titrations can be performed. Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. The equivalence point or end point of a coulometric. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Coulometric titrations are in many ways similar to volumetric. Equivalence Point In Coulometric Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Equivalence Point In Coulometric Titration Strong and weak acids and bases. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. The equivalence point or end point of a coulometric. An overview of possible titrations is given in. Equivalence Point In Coulometric Titration.
From www.pearson.com
The following pictures represent solutions at various stages in t Equivalence Point In Coulometric Titration The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. The equivalence point or end point of a coulometric. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a. Equivalence Point In Coulometric Titration.
From exyjcpegf.blob.core.windows.net
Titration And Equivalence Point at Bill Hubbard blog Equivalence Point In Coulometric Titration The equivalence point or end point of a coulometric. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. The concentration of the titrant is equivalent to the generating current, and the. Equivalence Point In Coulometric Titration.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Equivalence Point In Coulometric Titration Strong and weak acids and bases. An overview of possible titrations is given in table 1 [4]. Coulometric titrations are in many ways similar to volumetric titrations: Metric titrations can be performed. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Consider the reaction below in which fe 2 + is the. Equivalence Point In Coulometric Titration.
From philschatz.com
AcidBase Titrations · Chemistry Equivalence Point In Coulometric Titration Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. The. Equivalence Point In Coulometric Titration.
From www.researchgate.net
Coulometric titration curves for silver chalcogenides, with data taken Equivalence Point In Coulometric Titration An overview of possible titrations is given in table 1 [4]. The equivalence point or end point of a coulometric. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Let us consider the oxidation. Equivalence Point In Coulometric Titration.
From www.youtube.com
Coulometric Titration YouTube Equivalence Point In Coulometric Titration An overview of possible titrations is given in table 1 [4]. The equivalence point or end point of a coulometric. Consider the reaction below in which fe 2 + is the analyte and ce 4 + is the titrant. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Coulometric titrations are in. Equivalence Point In Coulometric Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Equivalence Point In Coulometric Titration Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. Coulometric titrations are in many ways similar to volumetric titrations: The equivalence point or end point of a coulometric. An overview of possible titrations is given in table 1 [4]. An examination of the two standard potentials for. Equivalence Point In Coulometric Titration.
From joiyrusdv.blob.core.windows.net
How To Find Titration Equivalence Point at Douglas Fuller blog Equivalence Point In Coulometric Titration Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. Coulometric titrations are in many ways similar to volumetric titrations: An examination of the two standard potentials for the. Equivalence Point In Coulometric Titration.
From mungfali.com
Equivalence Points On Titration Graph Equivalence Point In Coulometric Titration The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Coulometric titrations are in many ways similar to volumetric titrations: Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Strong and weak acids and bases. An overview of possible titrations is given in table. Equivalence Point In Coulometric Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Equivalence Point In Coulometric Titration An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. Coulometric titrations are in many ways similar to volumetric titrations: The equivalence point or end point of a coulometric. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to.. Equivalence Point In Coulometric Titration.
From solvedlib.com
What is a titration? What is the equivalence point o… SolvedLib Equivalence Point In Coulometric Titration An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. The equivalence point or end point of a coulometric. Consider the reaction below in which fe 2 + is the analyte and. Equivalence Point In Coulometric Titration.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Equivalence Point In Coulometric Titration An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision.. Equivalence Point In Coulometric Titration.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube Equivalence Point In Coulometric Titration An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Consider. Equivalence Point In Coulometric Titration.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Equivalence Point In Coulometric Titration Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Coulometric titrations are in many ways similar to volumetric titrations: The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. The present paper is a detailed account of the apparatus and techniques developed for coulometric. Equivalence Point In Coulometric Titration.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Equivalence Point In Coulometric Titration Metric titrations can be performed. Strong and weak acids and bases. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. The equivalence point or end point of a coulometric. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. Let us consider. Equivalence Point In Coulometric Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Equivalence Point In Coulometric Titration Strong and weak acids and bases. The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration.. Equivalence Point In Coulometric Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Equivalence Point In Coulometric Titration Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. Coulometric titrations are in many ways similar to volumetric titrations: Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. Strong and weak acids and bases. The equivalence point or end. Equivalence Point In Coulometric Titration.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Equivalence Point In Coulometric Titration Coulometric titrations are in many ways similar to volumetric titrations: Strong and weak acids and bases. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. An examination of the two standard potentials for the two half reactions confirms that in this pair cerium will. An overview of. Equivalence Point In Coulometric Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Equivalence Point In Coulometric Titration Coulometric titrations are in many ways similar to volumetric titrations: Coulometric titration (controlled current coulometry) a coulometric titration is an interesting variation on a classical redox titration. The equivalence point or end point of a coulometric. Metric titrations can be performed. An overview of possible titrations is given in table 1 [4]. The concentration of the titrant is equivalent to. Equivalence Point In Coulometric Titration.
From www.slideserve.com
PPT Coulometric Titration PowerPoint Presentation, free download ID Equivalence Point In Coulometric Titration The concentration of the titrant is equivalent to the generating current, and the volume of the titrant is. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change corresponds to. Coulometric titrations are in many ways similar to volumetric titrations: The present paper is a detailed account of the apparatus. Equivalence Point In Coulometric Titration.
From www.youtube.com
Titrations and the M1V1=M2V2 Math at The Equivalence Point YouTube Equivalence Point In Coulometric Titration An overview of possible titrations is given in table 1 [4]. Coulometric titrations are in many ways similar to volumetric titrations: The present paper is a detailed account of the apparatus and techniques developed for coulometric titrations of high precision. Let us consider the oxidation of fe2+ to fe3+ in coulometric titration, for each faraday of electricity the chemical change. Equivalence Point In Coulometric Titration.