Is Bromine Br2 . At room temperature and pressure, it is one of the few liquid elements. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Here is a collection of facts about the element: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is known for its brown color and characteristic acrid odor. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. Pure bromine is diatomic, br 2. Bromine is a halogen element with atomic number 35 and element symbol br.
from www.masterorganicchemistry.com
The most stable oxidation state of the element is −1, in which bromine occurs naturally. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Here is a collection of facts about the element: Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. Bromine is a halogen element with atomic number 35 and element symbol br. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Pure bromine is diatomic, br 2. Bromine is known for its brown color and characteristic acrid odor. At room temperature and pressure, it is one of the few liquid elements.
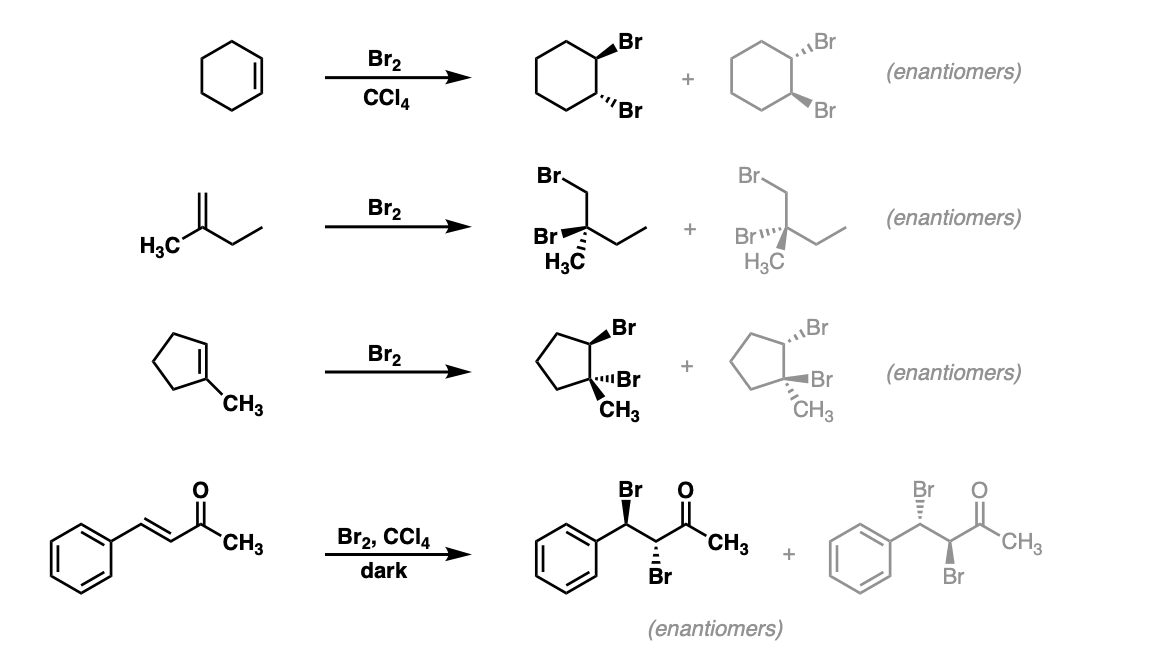
Bromination of alkenes with Br2 to give dibromides Master Organic
Is Bromine Br2 The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is known for its brown color and characteristic acrid odor. Here is a collection of facts about the element: Pure bromine is diatomic, br 2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is a halogen element with atomic number 35 and element symbol br. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. At room temperature and pressure, it is one of the few liquid elements.
From www.alamy.com
Elemental bromine (Br2), molecular model. Atoms are represented as Is Bromine Br2 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for. Is Bromine Br2.
From techiescientist.com
Is Br2 Polar or Nonpolar? Techiescientist Is Bromine Br2 Bromine is known for its brown color and characteristic acrid odor. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. At room temperature and pressure, it is one of the few liquid elements. Bromine is a halogen element with atomic number 35 and element symbol br. Pure bromine is diatomic, br 2. Br2, or bromine, is a diatomic molecule consisting of. Is Bromine Br2.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Is Bromine Br2 Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. Bromine is known for its brown color and characteristic acrid odor. The most stable oxidation state of the element is −1, in which bromine occurs naturally. At room temperature and pressure, it is one of the. Is Bromine Br2.
From www.dreamstime.com
Elemental Bromine Br2 Molecule. Skeletal Formula. Stock Illustration Is Bromine Br2 Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is known for its brown color and characteristic acrid odor. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. The most stable oxidation state. Is Bromine Br2.
From www.alamy.com
Bromine br hires stock photography and images Alamy Is Bromine Br2 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Pure bromine is diatomic, br 2. Here is a collection of facts about the element: Bromine is known for its brown color and characteristic acrid odor. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is. Is Bromine Br2.
From www.youtube.com
Alkenes Electrophilic Addition with Bromine, Br2, Bromination (Alevel Is Bromine Br2 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. At room temperature and pressure, it is one of the few liquid elements. Here is a collection of facts about the element: But oxidation states. Is Bromine Br2.
From www.alamy.com
Molecular Model of Bromine (Br2) Molecule. Vector Illustration Stock Is Bromine Br2 Bromine is known for its brown color and characteristic acrid odor. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Sources, facts, uses, scarcity. Is Bromine Br2.
From www.chegg.com
Solved Molecular bromine (Br2) at 25∘C is a liquid with a Is Bromine Br2 Bromine is known for its brown color and characteristic acrid odor. At room temperature and pressure, it is one of the few liquid elements. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Here is a. Is Bromine Br2.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Is Bromine Br2 Bromine is a halogen element with atomic number 35 and element symbol br. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. At room temperature and pressure, it is one of the few liquid elements. Pure bromine is diatomic, br 2. Here is a collection of facts about the. Is Bromine Br2.
From www.youtube.com
How to find the Oxidation Number for Br in Br2 (Bromine gas) YouTube Is Bromine Br2 But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Here is a collection of facts about the element: Pure bromine is diatomic, br 2. Br2, or bromine, is a diatomic molecule. Is Bromine Br2.
From fphoto.photoshelter.com
bromine phases transition chemistry element Fundamental Photographs Is Bromine Br2 Bromine is a halogen element with atomic number 35 and element symbol br. Pure bromine is diatomic, br 2. Here is a collection of facts about the element: At room temperature and pressure, it is one of the few liquid elements. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is known for its brown color and characteristic acrid odor.. Is Bromine Br2.
From pubs.acs.org
Bromine Radical (Br• and Br2•) Reactivity with Dissolved Organic Is Bromine Br2 At room temperature and pressure, it is one of the few liquid elements. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is a halogen element with atomic number 35 and element symbol. Is Bromine Br2.
From robhosking.com
14+ Br2 Lewis Structure Robhosking Diagram Is Bromine Br2 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Here is a collection of facts about the element: At room temperature and pressure, it is one of the few liquid elements. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. The most stable oxidation state of. Is Bromine Br2.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Is Bromine Br2 Pure bromine is diatomic, br 2. Here is a collection of facts about the element: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. At room temperature and pressure, it is one of the few liquid elements. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is the only nonmetallic element that is liquid. Is Bromine Br2.
From fphoto.photoshelter.com
bromine phases transition chemistry element Fundamental Photographs Is Bromine Br2 Bromine is a halogen element with atomic number 35 and element symbol br. Here is a collection of facts about the element: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is known for its brown color and characteristic acrid odor. The most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of. Is Bromine Br2.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Is Bromine Br2 Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. Bromine is a halogen element with atomic number 35 and element symbol br. Here is a collection of facts about the element: The most stable oxidation state of the element is −1, in which bromine occurs. Is Bromine Br2.
From www.alamy.com
Bromine molecular model hires stock photography and images Alamy Is Bromine Br2 Bromine is a halogen element with atomic number 35 and element symbol br. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. Here is a collection of facts about the element: The most stable oxidation state of the element is −1, in which bromine occurs. Is Bromine Br2.
From www.alamy.com
Bromine molecule Br2 Stock Photo Alamy Is Bromine Br2 Bromine is the only nonmetallic element that is liquid at ordinary temperatures. At room temperature and pressure, it is one of the few liquid elements. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is known for its brown color and characteristic acrid odor. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro. Is Bromine Br2.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image Is Bromine Br2 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. At room temperature and pressure, it is one of the few liquid elements. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a. Is Bromine Br2.
From www.vectorstock.com
Br2 bromine molecule Royalty Free Vector Image Is Bromine Br2 Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. At room temperature and pressure, it is one of the few liquid elements. Bromine is known for its brown color and characteristic acrid odor. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Pure bromine is diatomic,. Is Bromine Br2.
From www.alamy.com
Elemental bromine (Br2) molecule. Skeletal formula Stock Vector Image Is Bromine Br2 But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. At room temperature and pressure, it is one of the few liquid elements. Bromine is a halogen element with atomic number 35 and element symbol br. Pure bromine is diatomic, br 2. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is. Is Bromine Br2.
From stock.adobe.com
Molecular Model Of Bromine (Br2) Molecule. Vector Illustration. Stock Is Bromine Br2 But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is a halogen element with atomic number 35 and. Is Bromine Br2.
From cms.gutow.uwosh.edu
Bromine Is Bromine Br2 Pure bromine is diatomic, br 2. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is a halogen element with atomic number 35 and element symbol br. Bromine is known for its brown color and characteristic acrid odor. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5.. Is Bromine Br2.
From www.alamy.com
Elemental bromine hires stock photography and images Alamy Is Bromine Br2 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Here is a collection of facts about the element: Bromine is known for its brown color and characteristic acrid odor. Bromine is a halogen element with atomic number 35 and element symbol br. But oxidation states of. Is Bromine Br2.
From www.shutterstock.com
Bromine Br2 Alkaline Earth Metals Bromine Stock Illustration 2014218203 Is Bromine Br2 At room temperature and pressure, it is one of the few liquid elements. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Bromine is a halogen element. Is Bromine Br2.
From www.youtube.com
Reaction of Sodium (Na) with Bromine (Br2) YouTube Is Bromine Br2 Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is known for its brown color and characteristic acrid odor. Bromine is a halogen element with atomic number 35 and element symbol br. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and. Is Bromine Br2.
From www.chegg.com
Solved Electrophilic addition of bromine, Br2, to alkenes Is Bromine Br2 Pure bromine is diatomic, br 2. At room temperature and pressure, it is one of the few liquid elements. Bromine is known for its brown color and characteristic acrid odor. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Here is a. Is Bromine Br2.
From www.youtube.com
Is Br2 Polar or Nonpolar? (Bromine Gas) YouTube Is Bromine Br2 Pure bromine is diatomic, br 2. Here is a collection of facts about the element: Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. The most stable oxidation state of the element is. Is Bromine Br2.
From manuallistcantabank.z21.web.core.windows.net
Lewis Dot Diagram For Bromine Is Bromine Br2 Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. Here is a collection of facts about the element: At room temperature and pressure, it is one of the few liquid elements. Bromine is known for its brown color and characteristic acrid odor. But oxidation states. Is Bromine Br2.
From www.youtube.com
Is Br2 Polar or Nonpolar? Bromine Gas Polarity YouTube Is Bromine Br2 Here is a collection of facts about the element: But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Bromine is known for its brown. Is Bromine Br2.
From www.indiamart.com
Reagent Grade Liquid Bromine (Br2) at best price in Mumbai ID 1218994562 Is Bromine Br2 At room temperature and pressure, it is one of the few liquid elements. Bromine is known for its brown color and characteristic acrid odor. The most stable oxidation state of the element is −1, in which bromine occurs naturally. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry. Is Bromine Br2.
From www.dreamstime.com
Elemental Bromine Br2, Molecule. Skeletal Formula Stock Vector Is Bromine Br2 Bromine is the only nonmetallic element that is liquid at ordinary temperatures. Br2, or bromine, is a diatomic molecule consisting of two bromine atoms and is a powerful halogen used in organic chemistry for various reactions. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Pure bromine is diatomic, br 2.. Is Bromine Br2.
From www.youtube.com
Synthesis of Elemental Bromine (Br2) YouTube Is Bromine Br2 Here is a collection of facts about the element: At room temperature and pressure, it is one of the few liquid elements. Pure bromine is diatomic, br 2. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Bromine is known for its brown color and characteristic acrid odor. Bromine is a. Is Bromine Br2.
From www.youtube.com
How to Balance KI + Br2 = KBr + I2 (Potassium iodide + Bromine gas Is Bromine Br2 Pure bromine is diatomic, br 2. Bromine is known for its brown color and characteristic acrid odor. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Bromine is the only nonmetallic element that is liquid at ordinary temperatures. At room temperature and pressure,. Is Bromine Br2.
From www.youtube.com
Br2 (Bromine gas) Molecular Geometry, Bond Angles YouTube Is Bromine Br2 Here is a collection of facts about the element: The most stable oxidation state of the element is −1, in which bromine occurs naturally. But oxidation states of 0 (elemental bromine, br 2), +1 (hypobromite, bro −), +3 (bromite, bro −2), +5. At room temperature and pressure, it is one of the few liquid elements. Bromine is a halogen element. Is Bromine Br2.