Methyl Magnesium Bromide To Acetaldehyde . They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Grignard reagents are a source. (c) give balanced equations for the following reactions: What will happen if reacting acetaldehyde with methyl magnesium bromide. Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Here are some examples of reactions of grignards with aldehydes and ketones. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. 【solved】click here to get an answer to your question : (i) methyl magnesium bromide with ethyl alcohol. Here, i have a reaction. Note that in each case we are forming. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn.
from us.metoree.com
They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. (i) methyl magnesium bromide with ethyl alcohol. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Grignard reagents are a source. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. 【solved】click here to get an answer to your question : Here, i have a reaction. Here are some examples of reactions of grignards with aldehydes and ketones. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. What will happen if reacting acetaldehyde with methyl magnesium bromide.
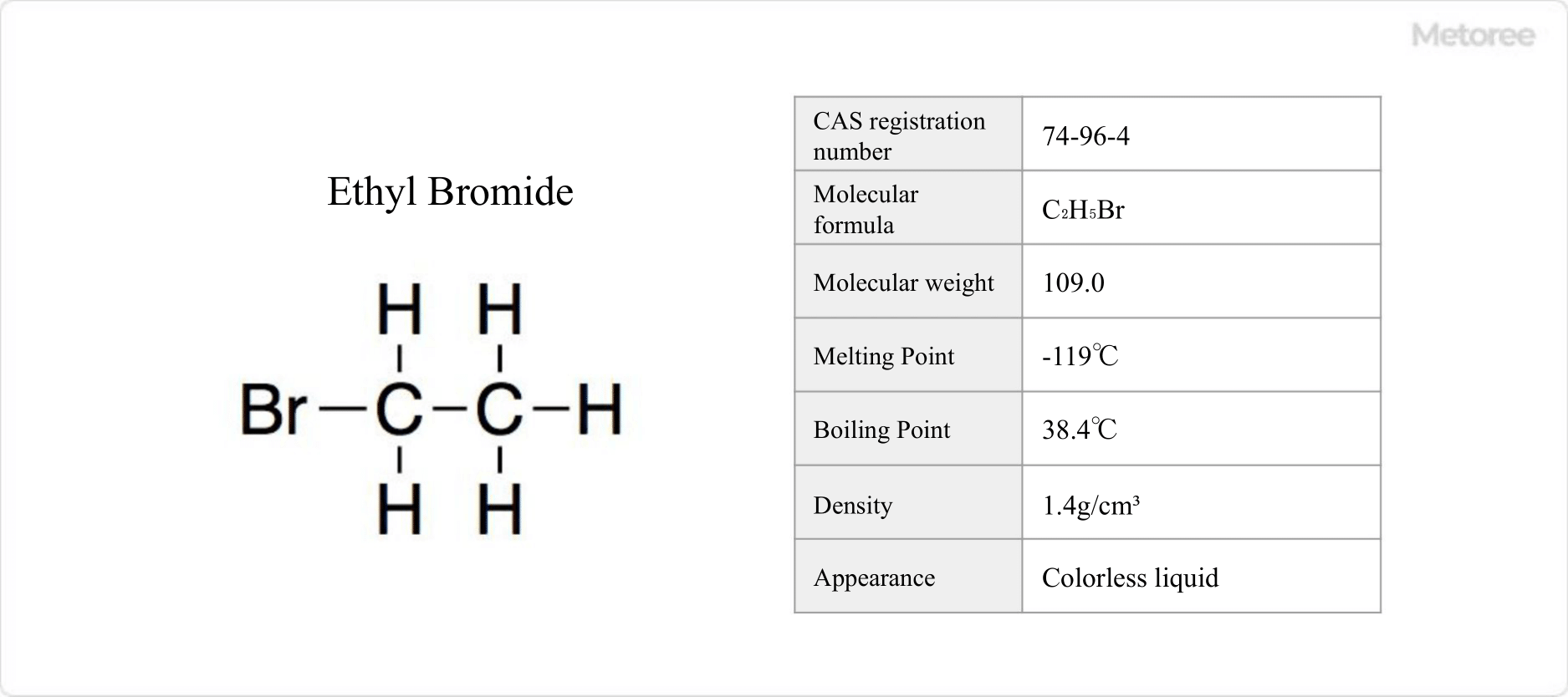
41 Ethyl Bromide Manufacturers in 2024 Metoree
Methyl Magnesium Bromide To Acetaldehyde Acetonitrile gives acetone when reacted with methyl magnesium iodide. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. 【solved】click here to get an answer to your question : Here are some examples of reactions of grignards with aldehydes and ketones. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. (c) give balanced equations for the following reactions: However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. Here, i have a reaction. Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Grignard reagents are a source. Note that in each case we are forming. What will happen if reacting acetaldehyde with methyl magnesium bromide. Acetonitrile gives acetone when reacted with methyl magnesium iodide. (i) methyl magnesium bromide with ethyl alcohol.
From www.toppr.com
What happens when methyl magnesium bromide is treated with formaldehyde Methyl Magnesium Bromide To Acetaldehyde Here are some examples of reactions of grignards with aldehydes and ketones. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Acetonitrile. Methyl Magnesium Bromide To Acetaldehyde.
From www.aluminummanufacturers.org
Aluminum Bromide Aluminum Sulfate Aluminum Manufacturers Methyl Magnesium Bromide To Acetaldehyde Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Here are some examples of reactions of grignards with aldehydes and ketones. 【solved】click here to get an answer to your question : Here, i have a reaction. Grignard reagents are a source. (c). Methyl Magnesium Bromide To Acetaldehyde.
From www.meritnation.com
2 Predict the product i)Ethanal + methyl magnesium chloride ii)Acetone Methyl Magnesium Bromide To Acetaldehyde Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. Grignard reagents are a source. Here are some examples of reactions of grignards with aldehydes and ketones. Note that in each case we are forming. Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Acetonitrile gives. Methyl Magnesium Bromide To Acetaldehyde.
From www.doubtnut.com
A reaction between methyl magnesium bromide and ethyl alcohol gives Methyl Magnesium Bromide To Acetaldehyde What will happen if reacting acetaldehyde with methyl magnesium bromide. (c) give balanced equations for the following reactions: Here, i have a reaction. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Here are some examples of reactions of grignards with aldehydes and ketones. Since the. Methyl Magnesium Bromide To Acetaldehyde.
From www.toptionchem.com
China Professional China Calcium Bromide Liquid Potassium Bromide Methyl Magnesium Bromide To Acetaldehyde Here, i have a reaction. What will happen if reacting acetaldehyde with methyl magnesium bromide. Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. (c) give balanced equations for the following reactions: Here are some examples of reactions of grignards with aldehydes and ketones. (i) methyl magnesium bromide with ethyl alcohol. However, aldehydes are. Methyl Magnesium Bromide To Acetaldehyde.
From www.toppr.com
CH3CHO [(ii) H2O](i) CH3MgBr (A) []H2SO4,Δ (B) [Oxidation]Hydroboration Methyl Magnesium Bromide To Acetaldehyde (i) methyl magnesium bromide with ethyl alcohol. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. What will happen if reacting acetaldehyde with methyl magnesium bromide. Here, i have a reaction. Here are some examples of reactions. Methyl Magnesium Bromide To Acetaldehyde.
From www.toppr.com
The reaction of methyl magnesium bromide reacts with acetone followed Methyl Magnesium Bromide To Acetaldehyde 【solved】click here to get an answer to your question : Note that in each case we are forming. Here, i have a reaction. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Here are some examples. Methyl Magnesium Bromide To Acetaldehyde.
From www.chemkits.eu
Sodium bromide, 99.8+, 7647156 Methyl Magnesium Bromide To Acetaldehyde Acetonitrile gives acetone when reacted with methyl magnesium iodide. What will happen if reacting acetaldehyde with methyl magnesium bromide. 【solved】click here to get an answer to your question : Grignard reagents are a source. Note that in each case we are forming. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and. Methyl Magnesium Bromide To Acetaldehyde.
From brainly.in
Formaldehyde when reacted with methyl magnesium bromide followed by Methyl Magnesium Bromide To Acetaldehyde They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Here are some examples of reactions of grignards with aldehydes and ketones. Grignard reagents are a source. Note that in each case we are forming. Grignard reagents are formed by the reaction. Methyl Magnesium Bromide To Acetaldehyde.
From www.chegg.com
Solved Draw the structure of the organic product(s) you Methyl Magnesium Bromide To Acetaldehyde Here, i have a reaction. 【solved】click here to get an answer to your question : What will happen if reacting acetaldehyde with methyl magnesium bromide. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. Grignard reagents are a source. Here are some examples of reactions of grignards with aldehydes and ketones.. Methyl Magnesium Bromide To Acetaldehyde.
From brainly.in
The product obtained on treating acetone with ethyl magnesium bromide Methyl Magnesium Bromide To Acetaldehyde Acetonitrile gives acetone when reacted with methyl magnesium iodide. Here, i have a reaction. 【solved】click here to get an answer to your question : However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. (i) methyl magnesium bromide with ethyl alcohol. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as. Methyl Magnesium Bromide To Acetaldehyde.
From molekula.com
Purchase Methylmagnesium bromide 1M in THF [75161] online • Catalog Methyl Magnesium Bromide To Acetaldehyde (c) give balanced equations for the following reactions: Here, i have a reaction. Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Grignard reagents are a source. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones.. Methyl Magnesium Bromide To Acetaldehyde.
From www.toppr.com
The reaction of methyl magnesium bromide with acetone followed by Methyl Magnesium Bromide To Acetaldehyde Here are some examples of reactions of grignards with aldehydes and ketones. (i) methyl magnesium bromide with ethyl alcohol. Note that in each case we are forming. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Here, i have a reaction. Since the grignard reagent is a strong nucleophile, it’ll. Methyl Magnesium Bromide To Acetaldehyde.
From www.maxhealth.co.nz
Glycopyrronium Bromide with Neostigmine Metisulfate Injection New Methyl Magnesium Bromide To Acetaldehyde 【solved】click here to get an answer to your question : Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Since the grignard reagent is a strong nucleophile, it’ll readily react with. Methyl Magnesium Bromide To Acetaldehyde.
From brainly.in
Convert methyl magnesium bromide to 2 methyl propane to all Brainly.in Methyl Magnesium Bromide To Acetaldehyde Acetonitrile gives acetone when reacted with methyl magnesium iodide. Note that in each case we are forming. (c) give balanced equations for the following reactions: Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. (i) methyl magnesium bromide with ethyl alcohol. Here, i have a reaction. Since the grignard reagent is a strong nucleophile,. Methyl Magnesium Bromide To Acetaldehyde.
From www.indiamart.com
22.00 Liquid Methyl Magnesium Chloride ( Cas No 676584), Analytical Methyl Magnesium Bromide To Acetaldehyde (i) methyl magnesium bromide with ethyl alcohol. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Here are some examples of reactions of grignards with aldehydes and ketones. Note that in each case we are forming. 【solved】click here to get an answer to your question : Grignard reagents are a source. Here, i have a reaction. Grignard reagents are formed. Methyl Magnesium Bromide To Acetaldehyde.
From www.universalmedicalinc.com
IBI IB40075 Ethidium Bromide Solution 10mL Methyl Magnesium Bromide To Acetaldehyde Grignard reagents are a source. (c) give balanced equations for the following reactions: 【solved】click here to get an answer to your question : Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. Grignard reagents are formed by. Methyl Magnesium Bromide To Acetaldehyde.
From www.drsebiscellfood.com
Bromide Plus Powder Dr. Sebi's Cell Food Methyl Magnesium Bromide To Acetaldehyde Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. 【solved】click here to get an answer to your question : They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. What will happen if reacting acetaldehyde with methyl magnesium bromide. Since the grignard reagent is a. Methyl Magnesium Bromide To Acetaldehyde.
From www.nagwa.com
Question Video Identifying Where Methyl Bromide Gas Is Commonly Found Methyl Magnesium Bromide To Acetaldehyde Here, i have a reaction. 【solved】click here to get an answer to your question : However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Here are some examples of reactions of grignards with aldehydes and ketones.. Methyl Magnesium Bromide To Acetaldehyde.
From www.toppr.com
Ethyl acetate is obtained when methyl magnesium bromide reacts with Methyl Magnesium Bromide To Acetaldehyde Note that in each case we are forming. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Acetonitrile gives acetone when reacted with methyl magnesium iodide. 【solved】click here to get an answer to your question : What will happen if reacting acetaldehyde with methyl magnesium bromide. (c) give balanced equations. Methyl Magnesium Bromide To Acetaldehyde.
From us.metoree.com
41 Ethyl Bromide Manufacturers in 2024 Metoree Methyl Magnesium Bromide To Acetaldehyde Acetonitrile gives acetone when reacted with methyl magnesium iodide. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. What will happen if reacting acetaldehyde with methyl magnesium bromide. Grignard reagents are a source. Here are some examples of reactions of grignards with aldehydes and ketones. Grignard reagents are formed by the. Methyl Magnesium Bromide To Acetaldehyde.
From www.toppr.com
4) CH, 88. Reaction between dry ice (solid CO,) and methyl magnesium Methyl Magnesium Bromide To Acetaldehyde Note that in each case we are forming. Here are some examples of reactions of grignards with aldehydes and ketones. 【solved】click here to get an answer to your question : Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Acetonitrile gives acetone when reacted with methyl magnesium iodide. However, aldehydes are obtained when grignard. Methyl Magnesium Bromide To Acetaldehyde.
From www.grainger.com
57090, 364.48, Cetyltrimethylammonium Bromide 6PLA0CE122500GM Methyl Magnesium Bromide To Acetaldehyde Acetonitrile gives acetone when reacted with methyl magnesium iodide. Here, i have a reaction. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. Note that in each case we are forming. (c) give balanced equations for the following reactions: 【solved】click here to get an answer to your question : (i) methyl. Methyl Magnesium Bromide To Acetaldehyde.
From askfilo.com
A= methyl magnesium bromide butyl ketone > Acetone > Acetaldehyde. SHORT Methyl Magnesium Bromide To Acetaldehyde 【solved】click here to get an answer to your question : (i) methyl magnesium bromide with ethyl alcohol. Here, i have a reaction. (c) give balanced equations for the following reactions: Note that in each case we are forming. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. Grignard reagents are formed by the reaction of. Methyl Magnesium Bromide To Acetaldehyde.
From www.indiamart.com
Sodium Bromide 45, Bromide salt of sodium, NaBr, 7647156, Sedoneural Methyl Magnesium Bromide To Acetaldehyde They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. (i) methyl magnesium bromide with ethyl alcohol. Note that in each case we are forming. 【solved】click here to get an answer to your question : (c) give. Methyl Magnesium Bromide To Acetaldehyde.
From www.toppr.com
What happens when formaldehyde is treated with methyl magnesium bromide Methyl Magnesium Bromide To Acetaldehyde They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. (c) give balanced equations for the following reactions: Here are some examples of reactions of grignards with aldehydes and ketones. Here, i have a reaction. What will happen if reacting acetaldehyde with methyl magnesium bromide. 【solved】click here to get an answer. Methyl Magnesium Bromide To Acetaldehyde.
From www.bhphotovideo.com
Photographers' Formulary Potassium Bromide (100g) 100930 100G Methyl Magnesium Bromide To Acetaldehyde Here, i have a reaction. Acetonitrile gives acetone when reacted with methyl magnesium iodide. (i) methyl magnesium bromide with ethyl alcohol. Grignard reagents are a source. Note that in each case we are forming. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. What will happen if reacting acetaldehyde with. Methyl Magnesium Bromide To Acetaldehyde.
From www.toppr.com
Which of the following are the intermediate in the reaction of excess Methyl Magnesium Bromide To Acetaldehyde What will happen if reacting acetaldehyde with methyl magnesium bromide. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Grignard reagents are a source. (c) give balanced equations for the following reactions: Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. (i) methyl magnesium bromide with ethyl alcohol. Here, i have. Methyl Magnesium Bromide To Acetaldehyde.
From brainly.in
Acetaldehyde is treated with Methyl Magnesium Iodide in the presence of Methyl Magnesium Bromide To Acetaldehyde Grignard reagents are a source. (c) give balanced equations for the following reactions: What will happen if reacting acetaldehyde with methyl magnesium bromide. Here are some examples of reactions of grignards with aldehydes and ketones. (i) methyl magnesium bromide with ethyl alcohol. Note that in each case we are forming. Acetonitrile gives acetone when reacted with methyl magnesium iodide. They’re. Methyl Magnesium Bromide To Acetaldehyde.
From www.coursehero.com
[Solved] Outline a balanced chemical equation for preparation of Methyl Magnesium Bromide To Acetaldehyde 【solved】click here to get an answer to your question : (i) methyl magnesium bromide with ethyl alcohol. Note that in each case we are forming. Grignard reagents are a source. Acetonitrile gives acetone when reacted with methyl magnesium iodide. Here, i have a reaction. Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. Here. Methyl Magnesium Bromide To Acetaldehyde.
From brainly.in
convert methyl magnesium bromide INTO 2 methyl propane 2ol Brainly.in Methyl Magnesium Bromide To Acetaldehyde Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. 【solved】click here to get an answer to your question : (i) methyl magnesium bromide with ethyl alcohol. Here, i have a reaction. Note. Methyl Magnesium Bromide To Acetaldehyde.
From www.rpicorp.com
E718001.0 Ethidium Bromide, Powder, 1 Gram Methyl Magnesium Bromide To Acetaldehyde Acetonitrile gives acetone when reacted with methyl magnesium iodide. They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Note that in each case we are forming. Grignard reagents are formed by the reaction of magnesium metal with alkyl or alkenyl halides. What will happen if reacting acetaldehyde with methyl magnesium. Methyl Magnesium Bromide To Acetaldehyde.
From pubs.acs.org
Magnesium Methyl CarbonateActivated Alkylation of Methyl Ketones with Methyl Magnesium Bromide To Acetaldehyde (c) give balanced equations for the following reactions: What will happen if reacting acetaldehyde with methyl magnesium bromide. 【solved】click here to get an answer to your question : They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Here are some examples of reactions of grignards with aldehydes and ketones. Grignard. Methyl Magnesium Bromide To Acetaldehyde.
From www.toppr.com
Which of the following are the intermediate in the reaction of excess Methyl Magnesium Bromide To Acetaldehyde Here, i have a reaction. Note that in each case we are forming. Since the grignard reagent is a strong nucleophile, it’ll readily react with electrophiles such as aldehydes and ketones. Grignard reagents are a source. However, aldehydes are obtained when grignard reagent are added with hydrogen cyanide , hcn. Acetonitrile gives acetone when reacted with methyl magnesium iodide. 【solved】click. Methyl Magnesium Bromide To Acetaldehyde.
From www.chegg.com
Solved o then H2O MgBr H OH 1.2 g ??? benzaldehyde Methyl Magnesium Bromide To Acetaldehyde They’re extremely good nucleophiles, reacting with electrophiles such as carbonyl compounds (aldehydes, ketones, esters, carbon dioxide, etc) and epoxides. Note that in each case we are forming. (c) give balanced equations for the following reactions: Here, i have a reaction. 【solved】click here to get an answer to your question : What will happen if reacting acetaldehyde with methyl magnesium bromide.. Methyl Magnesium Bromide To Acetaldehyde.