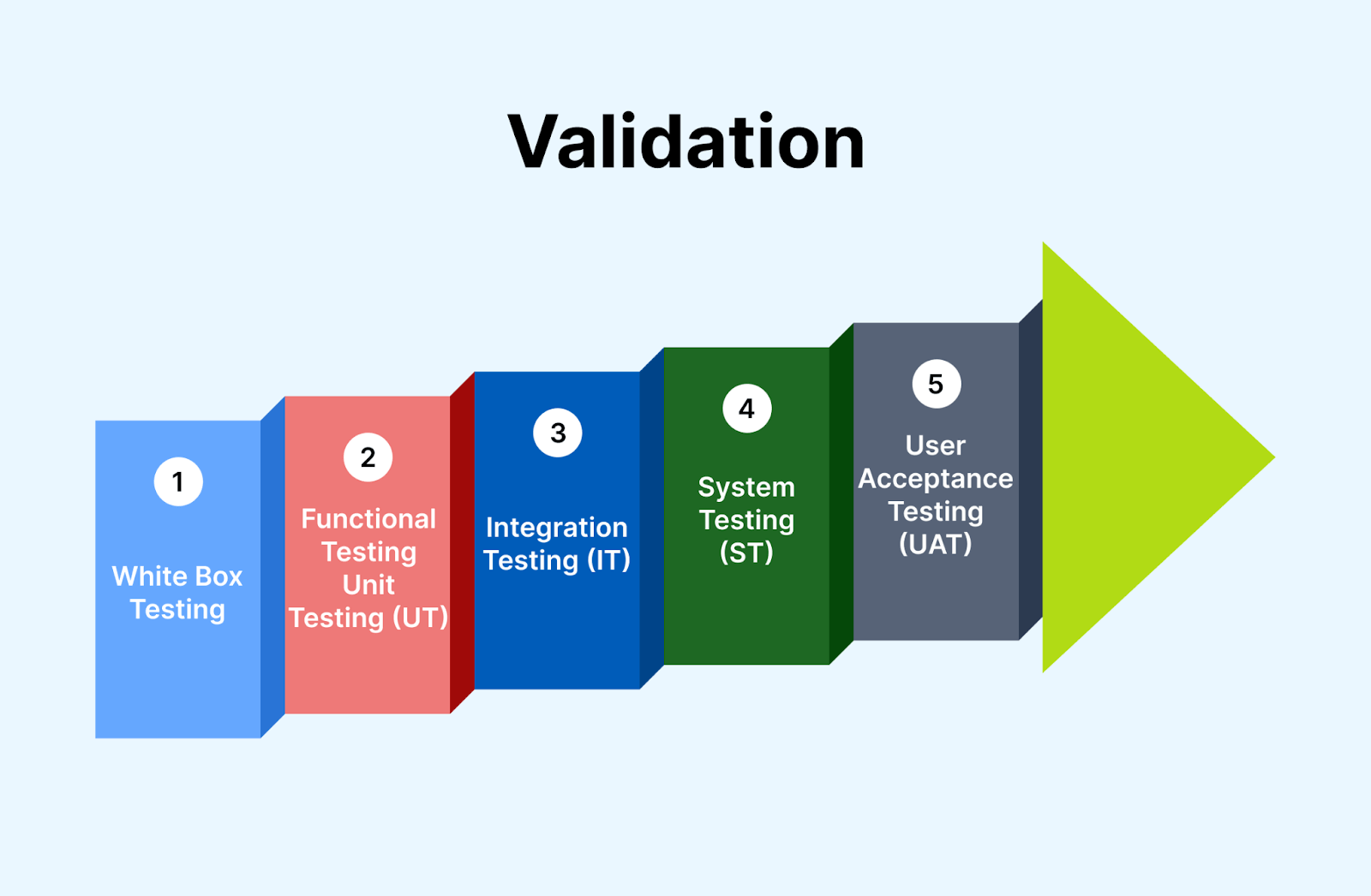
System Precision Method Validation . Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Consider the usp and ich. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system.
from www.lambdatest.com
In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Consider the usp and ich. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple.
Verification vs Validation Know The Differences in Testing
System Precision Method Validation In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Consider the usp and ich. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance.
From www.slideserve.com
PPT Analytical considerations PowerPoint Presentation ID707905 System Precision Method Validation Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Consider the usp and ich. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Precision (repeatability) precision of a method is the. System Precision Method Validation.
From www.researchgate.net
Precisionrecall curve of the four iterations of the crossvalidation... Download Scientific System Precision Method Validation Consider the usp and ich. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical methods. System Precision Method Validation.
From support.waters.com
Validation Protocols Setting up the System Precision Test Tip192 Waters System Precision Method Validation In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Typical. System Precision Method Validation.
From kvalito.ch
How is a system validated? Kvalito System Precision Method Validation Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. In the example provided in table 6, precision is determined for a number. System Precision Method Validation.
From www.scribd.com
Analytical Method Validation.pptx Verification And Validation Accuracy And Precision System Precision Method Validation Consider the usp and ich. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. In the example provided. System Precision Method Validation.
From mungfali.com
Test Method Validation System Precision Method Validation In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Typical system suitability parameters for. System Precision Method Validation.
From www.linkedin.com
HOW TO PERFORM ACCURACY DURING METHOD VALIDATION? System Precision Method Validation In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Consider the usp and ich. Validation of. System Precision Method Validation.
From www.scielo.br
SciELO Brazil VALIDATION OF ANALYTICAL METHODS IN A PHARMACEUTICAL QUALITY SYSTEM AN System Precision Method Validation Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical methods is required by. System Precision Method Validation.
From www.slideserve.com
PPT Accreditation & Validation PowerPoint Presentation, free download ID749120 System Precision Method Validation Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Consider the usp and ich.. System Precision Method Validation.
From mlros.com
Model Risk Management Model Validation The leading industry led forum for System Precision Method Validation Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Consider the usp and ich. In the. System Precision Method Validation.
From www.slideshare.net
analytical method validation and validation of hplc System Precision Method Validation In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Precision (repeatability) precision of a method is. System Precision Method Validation.
From www.slideserve.com
PPT ANALYTICAL METHOD VALIDATION PowerPoint Presentation, free download ID9092178 System Precision Method Validation Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Validation of analytical methods is required by. System Precision Method Validation.
From www.youtube.com
Validation of Analytical Method Linearity, Specificity, Range, Accuracy, Precision PART 1 System Precision Method Validation Consider the usp and ich. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Validation of analytical methods is required by most regulations and quality standards that impact laboratories.. System Precision Method Validation.
From www.youtube.com
VALIDATION OF ANALYTICAL METHOD Method validation Validation of an analytical procedure System Precision Method Validation Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Consider the usp and ich. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. In the example provided in table 6, precision is determined for a number of different levels during. System Precision Method Validation.
From www.chromatographyonline.com
Validation of StabilityIndicating HPLC Methods for Pharmaceuticals Overview, Methodologies System Precision Method Validation Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Consider the usp and ich. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system.. System Precision Method Validation.
From www.slideserve.com
PPT Exercise 7 Accuracy and precision PowerPoint Presentation, free download ID603162 System Precision Method Validation Consider the usp and ich. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Precision (repeatability). System Precision Method Validation.
From www.fyonibio.com
Assay Validation for and Biomarker Analysis FyoniBio System Precision Method Validation Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Consider the usp and ich. In the. System Precision Method Validation.
From www.lambdatest.com
Verification vs Validation Know The Differences in Testing System Precision Method Validation Consider the usp and ich. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical methods is required by most regulations and quality standards that impact laboratories.. System Precision Method Validation.
From www.slideshare.net
analytical method validation and validation of hplc System Precision Method Validation Consider the usp and ich. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Precision (repeatability) precision of a method. System Precision Method Validation.
From mes-global.com
Precision Vs Accuracy Example When It Comes To Sperm Analysis System Precision Method Validation Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Consider the usp and ich. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Precision (repeatability) precision of a method is the. System Precision Method Validation.
From www.slideshare.net
analytical method validation and validation of hplc System Precision Method Validation Consider the usp and ich. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical methods is required by most regulations and quality standards that impact laboratories.. System Precision Method Validation.
From support.waters.com
Validation Protocols Setting up the System Precision Test Tip192 Waters System Precision Method Validation Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical methods is required by most regulations. System Precision Method Validation.
From www.gmpsop.com
Concept of process Validation in Pharmaceutical Industry overview System Precision Method Validation Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Validation of analytical method (amv) is the. System Precision Method Validation.
From support.waters.com
Validation Protocols Setting up the System Precision Test Tip192 Waters System Precision Method Validation Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Consider the usp and ich. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Validation of analytical method (amv) is. System Precision Method Validation.
From www.slideserve.com
PPT Validation of pharmaceutical process, Analytical Method development Computer system System Precision Method Validation Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Consider the usp and ich. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. In the example provided in table 6, precision is determined for a number of different levels during. System Precision Method Validation.
From pharmagxp.com
Process Validation The Essential Guide to Ensuring Product Quality and Compliance Pharma GxP System Precision Method Validation Consider the usp and ich. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical methods is required by most regulations and quality standards that impact laboratories.. System Precision Method Validation.
From www.a3p.org
Some good validation practices for analytical procedures System Precision Method Validation Consider the usp and ich. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. In the. System Precision Method Validation.
From www.slideserve.com
PPT Validation of Analytical Methods PowerPoint Presentation, free download ID5719512 System Precision Method Validation Consider the usp and ich. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system.. System Precision Method Validation.
From www.slideserve.com
PPT An Introduction to Quality Assurance in Analytical Science PowerPoint Presentation ID System Precision Method Validation Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. In the example provided in table 6, precision is determined for a number. System Precision Method Validation.
From www.slideserve.com
PPT Instrumental Analysis PowerPoint Presentation, free download ID352128 System Precision Method Validation Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Typical system suitability parameters for chromatographic methods. System Precision Method Validation.
From www.gmpsop.com
What is analytical method validation in GMP System Precision Method Validation Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. Validation of analytical methods is required by most regulations and quality standards that impact laboratories. In the example provided in table 6, precision is determined for a number. System Precision Method Validation.
From www.youtube.com
Verification vs. Validation Precision vs. Accuracy YouTube System Precision Method Validation Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation. System Precision Method Validation.
From www.slideserve.com
PPT Instrumental Analysis PowerPoint Presentation, free download ID6603778 System Precision Method Validation Validation of analytical methods is required by most regulations and quality standards that impact laboratories. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Consider the usp and ich.. System Precision Method Validation.
From www.a3p.org
Some good validation practices for analytical procedures System Precision Method Validation Validation of analytical methods is required by most regulations and quality standards that impact laboratories. Typical system suitability parameters for chromatographic methods include (system) precision, tailing factor, theoretical. Consider the usp and ich. In the example provided in table 6, precision is determined for a number of different levels during validation, which include system. Validation of analytical method (amv) is. System Precision Method Validation.
From www.americanpharmaceuticalreview.com
Analytical Method Validation for Quality Assurance and Process Validation Professionals System Precision Method Validation Consider the usp and ich. Precision (repeatability) precision of a method is the closeness of agreement between a series of measurements obtained from multiple. Validation of analytical method (amv) is the process by which it is established, by laboratory studies, that the performance. In the example provided in table 6, precision is determined for a number of different levels during. System Precision Method Validation.