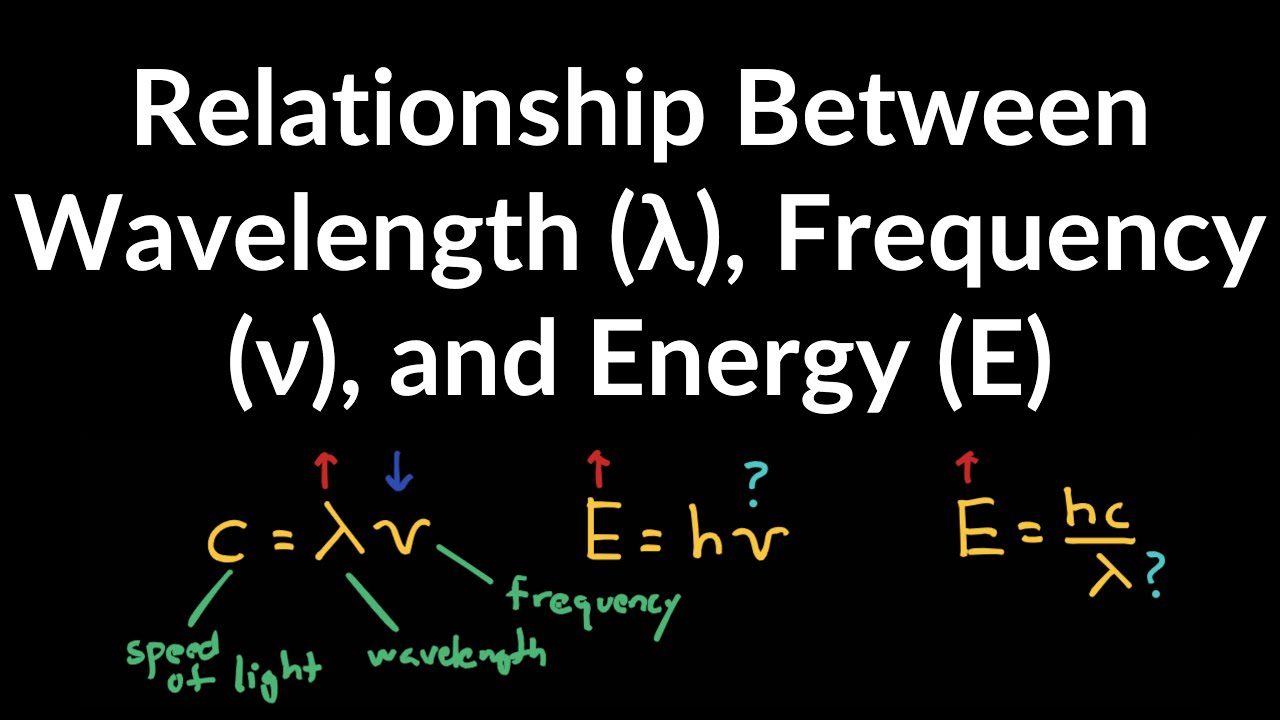
Energy Equation With Wavelength . E=hf=\frac {hc} {\lambda} e = hf = λhc. See examples of single photon and mole of photons energy with units and significant figures. — the energy of any body is related to its wavelength by the equation. The energy is simply the photon’s frequency multiplied by the planck constant (h). Where ‘h’ is a planck’s constant h=6.626. Frequency and wavelength are inverse correlated by way of the speed of light (c): H is the planck 's constant. — have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply. — the relationship between energy (e), frequency and wavelength can be described with this equation: — c = \nu \cdot \lambda c = ν ⋅ λ. E is the energy of the photon (in joules). — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. the energy of a photon can be calculated using the following formula: E = hc / λ.
from quizzlistachen.z13.web.core.windows.net
H is the planck 's constant. E is the energy of the photon (in joules). the energy of a photon can be calculated using the following formula: See examples of single photon and mole of photons energy with units and significant figures. E=hf=\frac {hc} {\lambda} e = hf = λhc. Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. — c = \nu \cdot \lambda c = ν ⋅ λ. The energy is simply the photon’s frequency multiplied by the planck constant (h). — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply.
Wavelength Frequency And Energy Equation
Energy Equation With Wavelength Where ‘h’ is a planck’s constant h=6.626. — the relationship between energy (e), frequency and wavelength can be described with this equation: E=hf=\frac {hc} {\lambda} e = hf = λhc. Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. the energy of a photon can be calculated using the following formula: The energy is simply the photon’s frequency multiplied by the planck constant (h). — have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? — c = \nu \cdot \lambda c = ν ⋅ λ. — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. Frequency and wavelength are inverse correlated by way of the speed of light (c): H is the planck 's constant. — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply. See examples of single photon and mole of photons energy with units and significant figures. Where ‘h’ is a planck’s constant h=6.626. E = hc / λ. — the energy of any body is related to its wavelength by the equation.
From www.studocu.com
Energy Wavelength Frequency Calculations Equations and constants Energy Equation With Wavelength The energy is simply the photon’s frequency multiplied by the planck constant (h). — the relationship between energy (e), frequency and wavelength can be described with this equation: See examples of single photon and mole of photons energy with units and significant figures. Frequency and wavelength are inverse correlated by way of the speed of light (c): Where ‘h’. Energy Equation With Wavelength.
From materialcampusfairchild.z21.web.core.windows.net
Wavelength Frequency And Energy Calculations Energy Equation With Wavelength E = hc / λ. Where ‘h’ is a planck’s constant h=6.626. — have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. — the relationship between energy (e),. Energy Equation With Wavelength.
From laurielee525.blogspot.com
Laurie Lee Chemistry Energy Equation With Wavelength Frequency and wavelength are inverse correlated by way of the speed of light (c): E is the energy of the photon (in joules). Where ‘h’ is a planck’s constant h=6.626. — the energy of any body is related to its wavelength by the equation. — with our wavelength to energy calculator, you can easily find the amount of. Energy Equation With Wavelength.
From classfullturfites.z13.web.core.windows.net
Calculating Frequency Wavelength And Energy Energy Equation With Wavelength — have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? — the relationship between energy (e), frequency and wavelength can be described with this equation: — c = \nu \cdot \lambda c = ν ⋅ λ. Where ‘h’ is a planck’s constant h=6.626. Frequency. Energy Equation With Wavelength.
From www.youtube.com
wavelength energy both formulas together YouTube Energy Equation With Wavelength the energy of a photon can be calculated using the following formula: Where ‘h’ is a planck’s constant h=6.626. See examples of single photon and mole of photons energy with units and significant figures. E = hc / λ. E is the energy of the photon (in joules). E=hf=\frac {hc} {\lambda} e = hf = λhc. H is the. Energy Equation With Wavelength.
From quizzlistachen.z13.web.core.windows.net
Wavelength Frequency And Energy Equation Energy Equation With Wavelength Frequency and wavelength are inverse correlated by way of the speed of light (c): See examples of single photon and mole of photons energy with units and significant figures. H is the planck 's constant. — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. — with our wavelength to. Energy Equation With Wavelength.
From www.youtube.com
Light equations solving for frequency (given wavelength) YouTube Energy Equation With Wavelength Frequency and wavelength are inverse correlated by way of the speed of light (c): See examples of single photon and mole of photons energy with units and significant figures. H is the planck 's constant. E is the energy of the photon (in joules). the energy of a photon can be calculated using the following formula: — have. Energy Equation With Wavelength.
From worksheetlistvis.z13.web.core.windows.net
Wavelength Frequency And Energy Equation Energy Equation With Wavelength the energy of a photon can be calculated using the following formula: — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. — c = \nu \cdot \lambda c = ν ⋅ λ. E=hf=\frac {hc} {\lambda} e = hf = λhc. Frequency and wavelength are inverse correlated by way. Energy Equation With Wavelength.
From www.youtube.com
Energy, Power and Intensity of Waves YouTube Energy Equation With Wavelength See examples of single photon and mole of photons energy with units and significant figures. the energy of a photon can be calculated using the following formula: E is the energy of the photon (in joules). H is the planck 's constant. E = hc / λ. — with our wavelength to energy calculator, you can easily find. Energy Equation With Wavelength.
From www.wikihow.com
How to Calculate Wavelength 10 Steps (with Pictures) Energy Equation With Wavelength the energy of a photon can be calculated using the following formula: Where ‘h’ is a planck’s constant h=6.626. The energy is simply the photon’s frequency multiplied by the planck constant (h). E = hc / λ. H is the planck 's constant. — c = \nu \cdot \lambda c = ν ⋅ λ. E is the energy. Energy Equation With Wavelength.
From mathformula5.netlify.app
How To Calculate The Speed Of An Wave Complete Guide Energy Equation With Wavelength Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. The energy is simply the photon’s frequency multiplied by the planck constant (h). — the relationship between energy (e), frequency and wavelength can be described with this equation: — learn how to calculate the energy of a photon from its wavelength. Energy Equation With Wavelength.
From twyfordphysicsy10.blogspot.com
GCSE Physics The Wave Equation Energy Equation With Wavelength E is the energy of the photon (in joules). See examples of single photon and mole of photons energy with units and significant figures. E=hf=\frac {hc} {\lambda} e = hf = λhc. Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. — the energy of any body is related to its. Energy Equation With Wavelength.
From sciencenotes.org
Wavelength to Frequency Calculation and Equation Energy Equation With Wavelength Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. Frequency and wavelength are inverse correlated by way of the speed of light (c): H is the planck 's constant. — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply. E =. Energy Equation With Wavelength.
From www.youtube.com
Wavelength Equation Part 1 YouTube Energy Equation With Wavelength — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply. — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. H is the planck 's constant. E=hf=\frac {hc} {\lambda} e = hf = λhc. — the energy of any. Energy Equation With Wavelength.
From byjus.com
for same energy find the ratio of wavelength of photon and wavelength Energy Equation With Wavelength Frequency and wavelength are inverse correlated by way of the speed of light (c): E is the energy of the photon (in joules). — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. Where ‘h’ is a planck’s constant h=6.626. the energy of a photon can be calculated using the. Energy Equation With Wavelength.
From learninglibrarythompson.z13.web.core.windows.net
Wavelength Frequency And Energy Equation Energy Equation With Wavelength E is the energy of the photon (in joules). H is the planck 's constant. — the energy of any body is related to its wavelength by the equation. Where ‘h’ is a planck’s constant h=6.626. Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. — c = \nu \cdot. Energy Equation With Wavelength.
From www.slideserve.com
PPT Waves PowerPoint Presentation, free download ID Energy Equation With Wavelength Where ‘h’ is a planck’s constant h=6.626. E=hf=\frac {hc} {\lambda} e = hf = λhc. Frequency and wavelength are inverse correlated by way of the speed of light (c): — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. — the energy of any body is related to its wavelength. Energy Equation With Wavelength.
From www.adda247.com
Energy formula in Physics & Equation for Class 10, 11 and 12 Energy Equation With Wavelength — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. E is the energy of the photon (in joules). — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply. H is the planck 's constant. The energy is simply the. Energy Equation With Wavelength.
From www.youtube.com
Energy from Wavelength Radiation Calculation YouTube Energy Equation With Wavelength — the relationship between energy (e), frequency and wavelength can be described with this equation: E=hf=\frac {hc} {\lambda} e = hf = λhc. Frequency and wavelength are inverse correlated by way of the speed of light (c): E = hc / λ. — learn how to calculate the energy of a photon from its wavelength using planck's and. Energy Equation With Wavelength.
From general.chemistrysteps.com
Calculating The Energy of a Photon Chemistry Steps Energy Equation With Wavelength H is the planck 's constant. E = hc / λ. — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. E=hf=\frac {hc} {\lambda} e = hf = λhc. The energy is simply the photon’s frequency multiplied by the planck constant (h). — have you the energy of two waves. Energy Equation With Wavelength.
From www.youtube.com
How To Calculate The Energy of a Photon Given Frequency & Wavelength in Energy Equation With Wavelength Where ‘h’ is a planck’s constant h=6.626. Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. — the energy of any body is related to its wavelength by the equation. E is the energy of the photon (in joules). E = hc / λ. See examples of single photon and mole. Energy Equation With Wavelength.
From worksheetauberges.z13.web.core.windows.net
How To Work Out The Wavelength Energy Equation With Wavelength — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply. Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. — learn how to calculate the energy of a photon from its wavelength using planck's and wave equations. — the. Energy Equation With Wavelength.
From www.youtube.com
Wavelength, Frequency, and Energy Practice Problems, Examples Energy Equation With Wavelength — c = \nu \cdot \lambda c = ν ⋅ λ. — have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? the energy of a photon can be calculated using the following formula: — learn how to calculate the energy of a photon. Energy Equation With Wavelength.
From questions.kunduz.com
1. What is the relationship between energy and wavel... Physics Energy Equation With Wavelength H is the planck 's constant. the energy of a photon can be calculated using the following formula: E=hf=\frac {hc} {\lambda} e = hf = λhc. — the energy of any body is related to its wavelength by the equation. — c = \nu \cdot \lambda c = ν ⋅ λ. Frequency and wavelength are inverse correlated. Energy Equation With Wavelength.
From meganchemblog.blogspot.com
Megan T's PreAP Chemistry Blog Energy, Wavelength, Speed Energy Equation With Wavelength H is the planck 's constant. — c = \nu \cdot \lambda c = ν ⋅ λ. — the energy of any body is related to its wavelength by the equation. — have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? See examples of. Energy Equation With Wavelength.
From www.slideserve.com
PPT Waves, & Their Speed, Derived from Maxwell’s Energy Equation With Wavelength E=hf=\frac {hc} {\lambda} e = hf = λhc. E is the energy of the photon (in joules). Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. See examples of single photon and mole of photons energy with units and significant figures. The energy is simply the photon’s frequency multiplied by the planck. Energy Equation With Wavelength.
From www.chegg.com
Solved of photons with given energy. Hence the wave length Energy Equation With Wavelength the energy of a photon can be calculated using the following formula: — the relationship between energy (e), frequency and wavelength can be described with this equation: Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s) and. — with our wavelength to energy calculator, you can easily find the amount. Energy Equation With Wavelength.
From www.youtube.com
WavelengthFrequency equation YouTube Energy Equation With Wavelength The energy is simply the photon’s frequency multiplied by the planck constant (h). — the energy of any body is related to its wavelength by the equation. — the relationship between energy (e), frequency and wavelength can be described with this equation: Here c c is the speed of light (299 792 458\ \text {m}/\text {s} 299792458 m/s). Energy Equation With Wavelength.
From whatsinsight.org
How to Calculate Energy With Wavelength? What's Insight Energy Equation With Wavelength — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply. E is the energy of the photon (in joules). — the energy of any body is related to its wavelength by the equation. The energy is simply the photon’s frequency multiplied by the planck constant (h). E=hf=\frac {hc}. Energy Equation With Wavelength.
From courses.lumenlearning.com
3.1 Energy General College Chemistry I Energy Equation With Wavelength — c = \nu \cdot \lambda c = ν ⋅ λ. — have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? the energy of a photon can be calculated using the following formula: E is the energy of the photon (in joules). E=hf=\frac {hc}. Energy Equation With Wavelength.
From www.youtube.com
Calculate the Energy, frequency & wavelength of an electron transition Energy Equation With Wavelength E=hf=\frac {hc} {\lambda} e = hf = λhc. the energy of a photon can be calculated using the following formula: Frequency and wavelength are inverse correlated by way of the speed of light (c): E is the energy of the photon (in joules). — the relationship between energy (e), frequency and wavelength can be described with this equation:. Energy Equation With Wavelength.
From haipernews.com
How To Find Energy Given Wavelength And Work Function Haiper Energy Equation With Wavelength E = hc / λ. Frequency and wavelength are inverse correlated by way of the speed of light (c): E is the energy of the photon (in joules). — have you the energy of two waves that have undergone a constructive or destructive interaction and want to find the new wavelength? H is the planck 's constant. See examples. Energy Equation With Wavelength.
From worksheetwolembavy.z21.web.core.windows.net
Wavelength Frequency And Energy Calculator Energy Equation With Wavelength — the energy of any body is related to its wavelength by the equation. — c = \nu \cdot \lambda c = ν ⋅ λ. The energy is simply the photon’s frequency multiplied by the planck constant (h). — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by. Energy Equation With Wavelength.
From materialfullnowadays.z13.web.core.windows.net
Energy Equation Wavelength Frequency Energy Equation With Wavelength E=hf=\frac {hc} {\lambda} e = hf = λhc. — the energy of any body is related to its wavelength by the equation. Frequency and wavelength are inverse correlated by way of the speed of light (c): — with our wavelength to energy calculator, you can easily find the amount of energy a photon carries by simply. H is. Energy Equation With Wavelength.
From www.youtube.com
Quantized Energy and Photons Chemistry Tutorial YouTube Energy Equation With Wavelength See examples of single photon and mole of photons energy with units and significant figures. — the energy of any body is related to its wavelength by the equation. Frequency and wavelength are inverse correlated by way of the speed of light (c): — learn how to calculate the energy of a photon from its wavelength using planck's. Energy Equation With Wavelength.