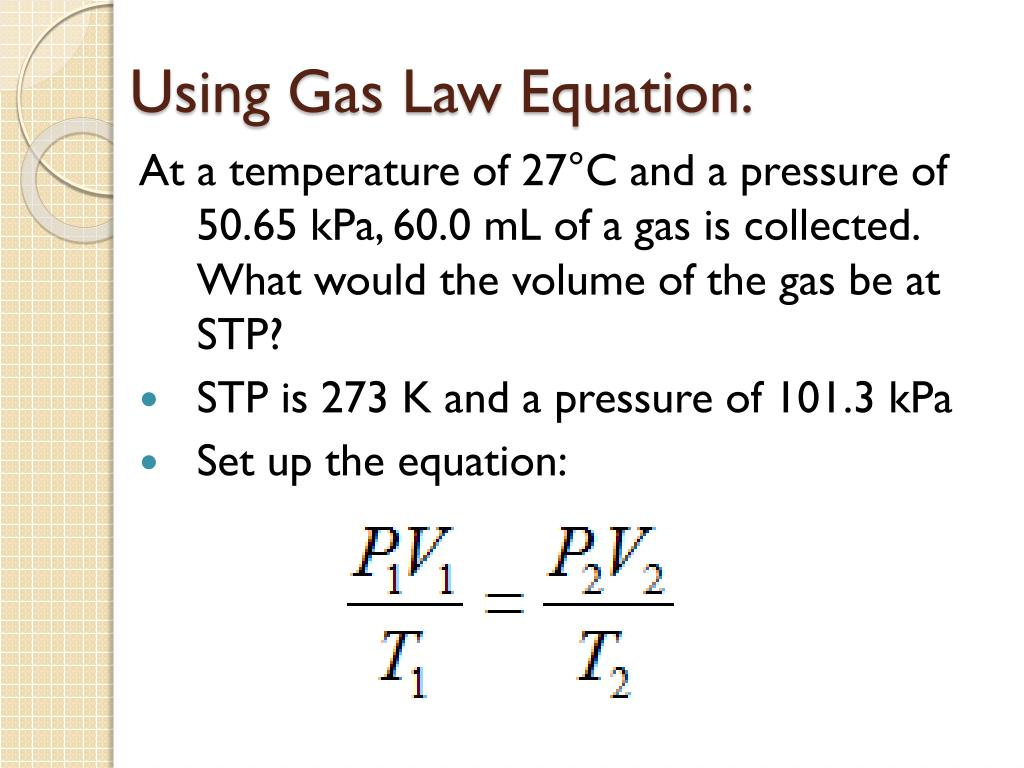
Help With Gas Law Problems . 1) what gas law should be used to solve this problem? What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. If gas at 75.0°c has a volume of 255 ml. One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. List the variables down the left side, solve the equation for the unknown variable and. Intro to gas laws student practice page instructions: A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Solve the equation for p 2. Notice that we have pressure, volume and temperature explicitly mentioned.
from www.slideserve.com
What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. If gas at 75.0°c has a volume of 255 ml. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. 1) what gas law should be used to solve this problem? List the variables down the left side, solve the equation for the unknown variable and. Intro to gas laws student practice page instructions: Solve the equation for p 2. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Notice that we have pressure, volume and temperature explicitly mentioned.
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985
Help With Gas Law Problems Solve the equation for p 2. If gas at 75.0°c has a volume of 255 ml. One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. 1) what gas law should be used to solve this problem? List the variables down the left side, solve the equation for the unknown variable and. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Solve the equation for p 2. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? Intro to gas laws student practice page instructions: A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Notice that we have pressure, volume and temperature explicitly mentioned.
From www.slideserve.com
PPT Gas Law Calculations PowerPoint Presentation, free download ID Help With Gas Law Problems What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? Notice that we have pressure, volume and temperature explicitly mentioned. Intro to gas laws student practice page instructions: What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Solve the. Help With Gas Law Problems.
From www.studocu.com
Ideal Gas Law Worksheet Ideal Gas Law Practice Worksheet Solve the Help With Gas Law Problems If gas at 75.0°c has a volume of 255 ml. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? Intro to gas laws student practice page instructions: Notice that we have pressure, volume and temperature explicitly mentioned. What would the volume of the gas be. Help With Gas Law Problems.
From www.youtube.com
Solving Combined Gas Law Problems YouTube Help With Gas Law Problems What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Notice that we have pressure, volume and temperature explicitly mentioned. Solve the equation for p 2. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? If gas at 75.0°c. Help With Gas Law Problems.
From www.slideserve.com
PPT Combined Gas Law PowerPoint Presentation, free download ID5864985 Help With Gas Law Problems A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Solve the equation for p 2. If gas at 75.0°c has a volume of 255 ml. Intro to gas laws student practice page instructions: What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. 1) what gas law should be. Help With Gas Law Problems.
From www.youtube.com
How to Solve Ideal Gas Law Problems (PV = nRT) YouTube Help With Gas Law Problems What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? Intro to gas laws student practice page instructions: 1) what gas law should be used to solve this problem? List the variables down the left side, solve the equation for the unknown variable and. What would. Help With Gas Law Problems.
From www.youtube.com
Solving Combined Gas Law Problems Charles' Law, Boyle's Law, Lussac's Help With Gas Law Problems Solve the equation for p 2. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Intro to gas laws student practice page instructions: One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. A gas has. Help With Gas Law Problems.
From www.youtube.com
Ideal Gas Law Practice Problems & Examples YouTube Help With Gas Law Problems Solve the equation for p 2. List the variables down the left side, solve the equation for the unknown variable and. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Notice that we have pressure, volume and temperature explicitly mentioned. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature. Help With Gas Law Problems.
From lessonmagicsapphist.z13.web.core.windows.net
Gas Law Problems With Answers Help With Gas Law Problems Notice that we have pressure, volume and temperature explicitly mentioned. List the variables down the left side, solve the equation for the unknown variable and. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Intro to gas laws student practice page instructions: Solve the equation for p 2. One way to state boyle’s law is. Help With Gas Law Problems.
From db-excel.com
Gas Law Problems Worksheet With Answers — Help With Gas Law Problems List the variables down the left side, solve the equation for the unknown variable and. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? If gas at 75.0°c has a volume of 255 ml. Solve the equation for p 2. Intro to gas laws student. Help With Gas Law Problems.
From www.slideserve.com
PPT II. Gas Laws PowerPoint Presentation, free download ID4136097 Help With Gas Law Problems If gas at 75.0°c has a volume of 255 ml. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Intro to gas laws student practice page instructions: 1) what gas law should be used to solve this problem? Solve. Help With Gas Law Problems.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Help With Gas Law Problems Intro to gas laws student practice page instructions: Solve the equation for p 2. List the variables down the left side, solve the equation for the unknown variable and. If gas at 75.0°c has a volume of 255 ml. One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to. Help With Gas Law Problems.
From www.youtube.com
Ideal Gas Law solution to problem 2 YouTube Help With Gas Law Problems One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? A gas has a volume of 800.0 ml at. Help With Gas Law Problems.
From www.showme.com
W34D4 combined gas law examples Science, Chemistry, Gases ShowMe Help With Gas Law Problems One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. 1) what gas law should be used to solve this problem? Intro to gas laws student practice page instructions: List the variables down the left side, solve the equation for the unknown variable. Help With Gas Law Problems.
From slidetodoc.com
Gas Laws and Nature of Gases Chapter 13 Help With Gas Law Problems If gas at 75.0°c has a volume of 255 ml. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. List the variables down the left side, solve the equation for the. Help With Gas Law Problems.
From www.youtube.com
Gas Law Practice Problems Boyle's Law, Charles' Law, Gay Lussac's Help With Gas Law Problems If gas at 75.0°c has a volume of 255 ml. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. List the variables down the left side, solve the equation for the unknown variable and. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Solve the equation for p. Help With Gas Law Problems.
From www.chegg.com
Solved Ideal Gas Law Practice Worksheet Solve the following Help With Gas Law Problems 1) what gas law should be used to solve this problem? If gas at 75.0°c has a volume of 255 ml. Solve the equation for p 2. Intro to gas laws student practice page instructions: Notice that we have pressure, volume and temperature explicitly mentioned. What would the volume of the gas be at 227.0 °c and 600.0 torr of. Help With Gas Law Problems.
From es.slideshare.net
Ideal Gas Law Problems Help With Gas Law Problems What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? Notice that we have pressure, volume and temperature explicitly mentioned. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. A gas has a volume of 800.0 ml at −23.0. Help With Gas Law Problems.
From www.youtube.com
Ideal Gas Law Practice Problems YouTube Help With Gas Law Problems List the variables down the left side, solve the equation for the unknown variable and. 1) what gas law should be used to solve this problem? What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. If gas at 75.0°c. Help With Gas Law Problems.
From printableroparstvaoy.z4.web.core.windows.net
Ideal Gas Law Practice Problems With Answers Help With Gas Law Problems What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Intro to gas laws student practice page instructions: Solve the equation for p 2. 1) what gas law should be used to solve this problem? What is the density of. Help With Gas Law Problems.
From studylib.net
2 Ideal Gas Laws Practice Help With Gas Law Problems What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? Notice that we have pressure, volume and temperature explicitly mentioned. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. List the variables down the left side, solve the equation. Help With Gas Law Problems.
From classcampusbar.z19.web.core.windows.net
Combined Gas Law Problems Worksheet Help With Gas Law Problems What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? 1) what gas law should be used to solve this problem? Intro to gas laws student practice page instructions: Solve. Help With Gas Law Problems.
From scienceducatio.weebly.com
Gas Laws Environmental Chemistry Help With Gas Law Problems 1) what gas law should be used to solve this problem? If gas at 75.0°c has a volume of 255 ml. List the variables down the left side, solve the equation for the unknown variable and. Intro to gas laws student practice page instructions: What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?.. Help With Gas Law Problems.
From studylib.net
General Gas Laws and Boyle's Law Practice Problems Help With Gas Law Problems If gas at 75.0°c has a volume of 255 ml. Notice that we have pressure, volume and temperature explicitly mentioned. Solve the equation for p 2. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k. Help With Gas Law Problems.
From printablefullstria.z19.web.core.windows.net
Gas Laws Worksheet With Answers Help With Gas Law Problems A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. 1) what gas law should be used to solve this problem? Solve the equation for p 2. Intro to gas laws student practice page instructions: One way to state boyle’s. Help With Gas Law Problems.
From www.slideserve.com
PPT Gas Law Calculations PowerPoint Presentation, free download ID Help With Gas Law Problems Solve the equation for p 2. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. List the variables down the left side, solve the equation for the unknown variable. Help With Gas Law Problems.
From www.pinterest.com
Gas Law Problems Worksheet Ideal gas law, Chemistry worksheets, Gas Help With Gas Law Problems 1) what gas law should be used to solve this problem? A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. If gas at 75.0°c has a volume of 255 ml. Intro to gas laws student practice page instructions: List the variables down the left side, solve the equation for the unknown variable and. What is. Help With Gas Law Problems.
From www.youtube.com
IDEAL GAS LAW PRACTICE PROBLEMS How to Solve Ideal Gas Law Problems Help With Gas Law Problems What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure of 113.0 kpa? Notice that we have pressure, volume and temperature explicitly mentioned. Intro to gas laws student practice page instructions: Solve the equation for p 2. List the variables down the left side, solve the equation for the. Help With Gas Law Problems.
From www.slideshare.net
Gas Law Sample Problems Help With Gas Law Problems 1) what gas law should be used to solve this problem? Intro to gas laws student practice page instructions: If gas at 75.0°c has a volume of 255 ml. Notice that we have pressure, volume and temperature explicitly mentioned. What is the density of laughing gas, dinitrogen monoxide, n 2 o, at a temperature of 325 k and a pressure. Help With Gas Law Problems.
From www.youtube.com
Combined Gas Law Problems YouTube Help With Gas Law Problems What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Solve the equation for p 2. List the variables down the left side, solve the equation for the unknown variable and. If gas at 75.0°c has a volume of 255 ml. 1) what gas law should be used to solve this problem? A gas. Help With Gas Law Problems.
From studylib.net
Gas Law Problems Help With Gas Law Problems Notice that we have pressure, volume and temperature explicitly mentioned. One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Solve the equation for p 2. 1) what gas. Help With Gas Law Problems.
From studylib.net
Combined Gas Law Problems Help With Gas Law Problems 1) what gas law should be used to solve this problem? List the variables down the left side, solve the equation for the unknown variable and. Intro to gas laws student practice page instructions: Notice that we have pressure, volume and temperature explicitly mentioned. One way to state boyle’s law is “all other things being equal, the pressure of a. Help With Gas Law Problems.
From www.youtube.com
Solving Ideal Gas Law Problems Calculate Volume (Part 2, Q2) YouTube Help With Gas Law Problems If gas at 75.0°c has a volume of 255 ml. Intro to gas laws student practice page instructions: A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. What is the density of laughing gas, dinitrogen monoxide, n 2 o,. Help With Gas Law Problems.
From study.com
Quiz & Worksheet Ideal Gas Law Practice Problems Help With Gas Law Problems What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Intro to gas laws student practice page instructions: Notice that we have pressure, volume and temperature explicitly mentioned. Solve the equation for p 2. List the variables down the left side, solve the equation for the unknown variable and. A gas has a volume. Help With Gas Law Problems.
From www.youtube.com
Gas Law Practice Problems YouTube Help With Gas Law Problems 1) what gas law should be used to solve this problem? One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. What would the volume of the gas be at 227.0 °c and 600.0 torr of pressure?. Solve the equation for p 2.. Help With Gas Law Problems.
From learninglibrarydon.z13.web.core.windows.net
Gas Law Problems With Answers Help With Gas Law Problems A gas has a volume of 800.0 ml at −23.0 °c and 300.0 torr. Solve the equation for p 2. One way to state boyle’s law is “all other things being equal, the pressure of a gas is inversely proportional to its volume.” (a) what is the. What would the volume of the gas be at 227.0 °c and 600.0. Help With Gas Law Problems.